Bioenergetics PowerPoint PPT Presentation
1 / 59
Title: Bioenergetics
1
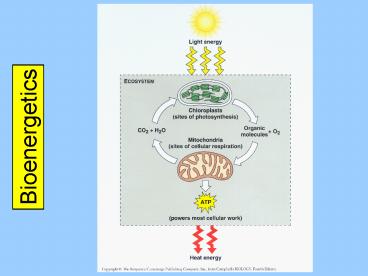
Bioenergetics
2
Oxidation and Reduction
- Oxidation is the Loss of Electrons
- E.g., something that is oxidized in the course of
a chemical reaction with Oxygen has had electrons
stolen by Oxygen - Reduction is the Gain of Elections
- E.g., a gain of electrons results in a decrease
(reduction) in electrical charge (since electrons
carry a negative charge) - Metal ores are Reduced to metals (via the
addition of electrons)metals found in ores are
in an oxidized form relative to metals found as
metals
3
Oxidation and Reduction
- Note that oxidation and reduction are not
necessarily complete - E.g., movement of an electron from relatively
close to an atoms nucleus to farther away, but
still bonded, is also oxidation - (and moving an electron closer is still
reduction) - E.g., electrons in C-H bond are closer to C than
those in C-O bond
4
Oxidation of Carbon (1/2)
5
Oxidation of Carbon (2/2)
H H H-C-O H
O-H H-CO
H H-CO
H H-C-H H
OCO
This is Carbon Dioxide
6
Complete Oxidation of a Hydrocarbon
CXHY (X¼Y)O2 ? XCO2 ½Y(H2O)
Energy
- Note that each Carbon gives rise to one CO2
- Note that every 2 Hydrogens gives rise to one H2O
- CO2 is the common highly Oxidized form of Carbon
- H2O is the common highly Oxidized form of
Hydrogen - (note also that H2O represents a reduced form of
Oxygen)
C3H8 (32)O2 ? 3CO2 4(H2O)
Energy
7
Complete Oxidation of Glucose
CXHY (X¼Y)O2 ? XCO2 ½Y(H2O)
Energy
C6H12O6 (63-3)O2 ? 6CO2 6(H2O)
Glucose is a Hexose!
C6H12O6 6O2 ? 6CO2 6(H2O)
Energy
8
Oxidizing Agents (e.g., NAD)
9
Oxidizing Agents (e.g., FAD)
10
Oxidation and Reduction II
- Recall that both FAD and NAD can oxidize other
molecules - In doing so they remove two electrons and two
protons - In the process FAD is reduced to FADH2 and NAD
to NADH H - Note that two electrons two protons (I.e., 2H)
two Hs - (that is, neutral Hydrogen atoms)
- Note that FADH2 NADH H can be oxidized
- In the course of this oxditation they are reduced
11
Reducing Agents (e.g., NADH)
- Oxidizing agents steal electrons
- In the process they are reduced
- Reducing agents donate electrons
- In the process they are oxidized
12
Pop Quiz!
FADH2
So which is the Reducing agent, FAD or FADH2?
13
Energy (another reminder)
Potential Energy
Kinetic Energy
Kinetic
14
C H Oxidation Releases Energy
Oxidation is the movement of electrons from near
the nucleus of certain atoms (e.g., Carbon or
Hydrogen) to even closer to the nucleus of
another atom (e.g., Oxygen) The oxidation of
atoms such as carbon or hydrogen therefore can
liberate energy This liberated energy can be
harnessed
15
Dehydrogenases
- Oxidation and Reduction in biological systems
typically is catalyzed by enzymes - Oxidation mediated by the coenzyme NAD is
catalyzed by enzymes know as Dehydrogenases - Note that these are de-Hydrogen-ases these are
enzymes that catalyze the removal of Hydrogen
atoms - The general reaction in which NAD participates
is - NAD 2H 2e- ? NADH H
- which is equivalent to
- NAD 2H ? NADH H
16
Dehydrogenases
- H-C-O-H NAD dehydrogenase ?
- CO NADH H dehydrogenase
- Note that the dehydrogenase is found on both
sides of the equation it is a catalyst so is not
used up - 2H-C-C-H2 FAD dehydrogenase ?
- H-CC-H FADH2 dehydrogenase
- Note in both reactions the loss of two hydrogen
atoms
17
Glyceraldehyde-3-Phosphate Dehydrogenase
enzyme
18
ATP Energy Currency of Cells
- - -
-
19
Cellular Respiration, Overview
20
ATP-Producing Pathways
Glycolysis
Cellular Respiration
Photosynthesis
21
Oxidative vs. Substrate-Level Phosphorylation
- These are concepts whose distinction may not make
sense to you until weve covered the entire
chapter - Substrate-Level Phosphorylation is donation of
phosphate to ADP that is directly powered by
making breaking bonds - Substrate ? Product (Energy) ADP Pi ? ATP
- Oxidative Phosphorylation powered by a
Proton-Motive Force - There are a variety of ways to produce a
Proton-Motive Force, all more complicated than
Substrate-Level Phosphorylation - These ways typically involving Electron Transport
22
Glycolyis in Detail
23
Glycolyis in Detail
24
Glycolyis in Detail
25
Glycolyis in Detail
26
Glycolyis in Detail
27
Outline of Glycolysis
An Enzyme- and Coenzyme-mediated catabolic pathway
28
Synopsis of Glycolysis
- C6 (a.k.a., glucose) ATP ? C6-P ADP
- C6-P ATP ? P-C6-P ADP
- P-C6-P ? 2C3-P (this is the sugar-splitting step)
- (note the stoichiometry of all of the following
are 2 for every one glucose) - C3-P NAD Pi ? P-C3-P NADH H
- P-C3-P ADP ? C3-P ATP
- C3-P ADP ? C3 (a.k.a., pyruvate) ATP
This is the minimal level at which you must learn
the steps of glycolysis
29
Substrate-Level Phosphorylation
30
Substrate-Level Phosphorylation
31
Bioenergetics
32
Mitochondrial Reactions
33
Pyruvate Oxidation
34
Acetyl CoA
Coenzyme A
acetyl
35
Oxaloacetate ? Citrate
a.k.a., Citric Acid
a.k.a., Tricarboxylic Acid
36
Krebs Citric Acid Cycle
37
Krebs Citric Acid Cycle
Do you see the error in this figure?
- Note that these are per Acetyl-CoA
- That means two turns of Krebs cycle per Glucose
38
Krebs Citric Acid Cycle
citric acid
oxaloacetate
citrate
39
Bioenergetics
40
Electron Transport Chain
41
Electron Transport Chain
42
Electron Transport Chain
H
H
H
H
Note generation of Proton Motive Force
43
Oxidative vs. Substrate-Level Phosphorylation
44
Reverse-Running H Pump
45
ATP Bookkeeping
glycolysis
pyruvate oxidation
Krebs cycle
46
ATP Bookkeeping
- One glucose yields
- 2 ATP in glycolysis
- 2 NADH in glycolysis
- 2 NADH as pyruvate enters citric acid cycle
- 2 ATP in citric acid cycle
- 6 NADH in citric acid cycle
- 2 FADH2 in citric acid cycle
47
ATP Bookkeeping
48
Anaerobic Respiration
49
Anaerobic Respiration
employs an inorganic molecule other than O2 as a
terminal electron acceptor.
50
Glycolysis NAD Requirement
?
?
?
?
?
51
Aerobic NAD Regeneration
52
Anaerobic Regeneration
Fermenation Pathways
53
Homolactic Acid Fermentation
54
Alcoholic Fermentation
55
Alcoholic Fermentation
No, These are Not Lemons!
56
Mixed-Acid Fermentation (e.g., E. coli)
57
Link to Next Presentation
58
Acknowledgements
http//www.life.uiuc.edu/biochem/352/lecture_28/le
cture_28.ppt http//ibscore.dbs.umt.edu/bio221/dow
nloadnotes/Biol221_24a.ppt http//207.233.44.253/w
ms/reynolmj/lifesciences/lecturenote/bio3/Chap06.p
pt
59
Glyceraldehyde-3-Phosphate Dehydrogenase