Ozone - PowerPoint PPT Presentation
1 / 29
Title:
Ozone
Description:
correctly predicted a US oil production peak in 1970. USA Lower 48 Oil Discovery and Production ... enough so that a CO2-water clathrate might form... – PowerPoint PPT presentation
Number of Views:143
Avg rating:3.0/5.0
Title: Ozone
1
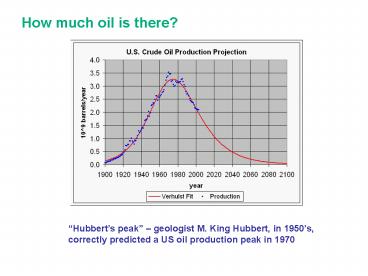
How much oil is there?
Hubberts peak geologist M. King Hubbert, in
1950s, correctly predicted a US oil production
peak in 1970
2
USA Lower 48 Oil Discovery and Production
Oil depletion in the US shows a 42 year gap
between peak discovery and peak production. No
new large fields found since early 1980s. As
goes the US, so goes the world?
3
Worldwide growing gapbetween oil discovery and
production
Green vertical bars depict years where
Discoveries exceeded Production. The red bars
show years where Discoveries were less than
Production Current ratio 4 barrels consumed
for every new barrel of oil found
4
World Conventional Oil
Global oil production has been essentially flat
since about 2000 The world is on a plateau
where the US alone was in about 1970
5
All Hydrocarbons Peak
6
Total Barrels
Alternative view CERA Cambridge Energy
Research Associates Claim 3x greater reserves
than most other analysts Optimism that much
more of the resource will become a reserve
7
The Hydrocarbon Age
8
Some statistics on conventional oil Total world
conventional oil reserves in 1780 2 trillion
barrels (2 x 1012) 1780 Watt develops the steam
engine Industrial Revolution starts Total world
conventional oil reserves remaining (2005) 1
trillion barrels Yearly rate of world oil
consumption 31 billion barrels (31 x 109) Daily
rate 85 million barrels burned per
day Consumption in the United States US burns
25 of all the oil (and all energy use) in the
world US of total world population (6.6
billion) 4.6 Should we drill in the Arctic
National Wildlife Refuge? Alaskan oil yields
most optimistic estimates are for 1 million
barrels per day starting in about 10
years Present US consumption 18 million barrels
per day Potential net gain 6 increase in oil
availability Potential net loss Alaskan
environmental damage
9
(No Transcript)
10
Coal-Derived Fuels
-make the coal cleaner by increasing the H/C
ratio Hydrogasification C 2 H2 ? CH4 T
800C DH -75 kJ/mol At high T, reaction
proceeds in the reverse direction Methanation of
CO CO 3 H2 ? CH4 H2O T 400C Ni
catalyst DH -206 kJ/mol Production of liquid
fuels from CO (Fischer-Tropsch chemistry) nCO
(2n 1)H2 ? CnH2n2 nH2O Production of
methanol from CO CO 2 H2 ? CH3OH Where do
the CO and H2 come from?
11
Coal-Derived Fuels
Production of CO and H2 from coal Steam
reforming C H2O ? CO H2 T 900C DH
131 kJ/mol Equimolar CO and H2 are
produced Water-gas shift reaction CO H2O ?
CO2 H2 DH -41 kJ/mol Produces additional
hydrogen gt21 mole ratio of H2CO needed to
produce liquid fuels This chemistry was used by
Germany in WWII when the Allies cut off oil
shipments into the country.
12
Coal-Derived Fuels
- Combining these reactions
- CO 3 H2 ? CH4 H2O (methanation of CO DH
-206.3 kJ/mol) - 2C 2H2O ? 2CO 2H2 (steam reforming DH
131.4 kJ/mol)) - CO H2O ? CO2 H2 (water-gas shift
reaction DH -41.4 kJ/mol) - 2C 2 H2O ? CO2 CH4 (combining reactions)
- Overall DH 15 kJ/mol
- ? all the heating value of coal can be
transferred to CH4 - with only 15 kJ/mol energy expenditure (in
theory) - Unfortunately the reactions are poorly matched
heat derived - from methanation cant drive steam reforming
because the latter - needs an extremely high temperature
- So steam reforming is driven by burning more coal
- Energy efficiency of the overall process is
lowered - Greenhouse effect is larger than producing the
same energy from coal alone
13
Production of hydrogen (and food)
- Can be accomplished from oil and gas as well as
coal - CH4 2 H2O ? 4 H2 CO2 (hydrogen gas from
methane reforming) - This is the major route to H2 today
- Fossil fuel production of H2 is used to produce
ammonia (Haber process) - N2 3 H2 ? 2 NH3 from thin air
- Then NH3 2 O2 ? HNO3 H2O
- NH3 HNO3 ? NH4NO3
- to produce fertilizers
- Food production at levels needed to sustain
current population is - presently almost fully dependent on nonrenewable
fossil fuel
14
Emissions in the United States
CO2 Emissions From Coal-Fired Electricity
Generation 1897 billion tons
31.7
Other Emissions 3675 billion tons
CCS is a viable strategy in the stationary
power plants, not for the mobile sources of
CO2
CO2 Emissions From Other Electricity
Generation 416 billion tons
U.S. Total CO2 Emissions 5,988 billion tons
(2004)
15
Capture and Geologic Storage of CO2 Avoids
Emissions
- CO2 is scrubbed from the smoke stack emissions
- CO2 is injected deep underground
A Four Step Process
Capture
Compression
Underground Injection
Pipeline Transport
16
Options for CO2 Capture
- Post-combustion
- Established technology
- Pre-combustion
- Established technology for other applications
- Not demonstrated for power production
- Oxygen combustion
- Not demonstrated for power production
Burning directly in oxygen produces only CO2
and water but too hot for available materials
17
Options for CO2 Capture
- Post-combustion
- Conventional pulverized coal plant
- Burn coal in air
- Exhaust is mostly N2 and 15 CO2
- Scrubbing of CO2 uses amines
- R2NH (l) CO2 ? R2NCOO- H
- The CO2 enters the liquid phase
- The amine liquid is then separated and heated to
release the concentrated CO2 for capture - Requires retrofitting of existing plants
18
Options for CO2 Capture
- Pre-combustion
- IGCC integrated gasification
- combined cycle
- Gasify the coal first produces
- syngas CO H2
- Water shift reaction to generate
- CO2
- Remove SO2, other impurities
- Remove CO2 with amine reaction
- Burn very clean H2
19
Where to store the carbon? (at least several
GtC/yr) -deep ocean burial -very deep
aquifers -depleted oil/gas reservoirs
Dissolving CO2 in seawater CO2 (g) H2O ?
H2CO3 ? H HCO3- Ocean acidic means
depositing CO2 sufficiently offshore and deep
enough so that a CO2-water clathrate might form
Ocean neutral is better react CO2 with CaCO3
or CaSiO3 CO2 (g) H2O CaCO3 (s) ?
Ca(HCO3)2 (aq) --traps CO2 so it will not
ultimately escape (if at ocean bottom)
20
Options for Geological Storage
- Oil and gas fields
- Depleted
- EOR, EGR
- Saline formations
- Unminable coal-seams
From IPCC Special Report
21
What Keeps the CO2 Underground?
Ground Surface
- Injected at depths of 1 km or deeper into rocks
with tiny pore spaces - Primary trapping
- Beneath seals of low permeability rocks
- Secondary trapping
- CO2 dissolves in water
- CO2 is trapped by capillary forces
- CO2 converts to solid minerals
Sand
Shale
Sandstone
Shale
Sandstone
Shale (seal)
1/10 inch
Storage security increases over time due to
secondary trapping mechanisms.
Sandstone (storage formation)
22
Multiple Lines of Evidence Indicate Storage Can
Be Secure and Effective
- Natural analogues
- Oil and gas reservoirs
- CO2 formations
- Industrial analogues
- CO2 EOR
- Natural gas storage
- Liquid waste disposal
- Existing projects
- Sleipner, Off-shore Norway
- Weyburn, Canada
- In Salah, Algeria
470 natural gas storage facilities in the U.S.
20 to 30 Mt/yr are injected for CO2-EOR
23
Capacity of Storage Formations
a. Estimates would be 25 larger if undiscovered
reserves were included.
From IPCC Special Report
Available evidence suggests that worldwide, it
is likely that there is a technical potential of
at least about 2,000 GtCO2 (545 GtC) of storage
capacity in geological formations.
24
(No Transcript)
25
Nuclear energy binding curve
Basis for fission energy
Basis for fusion energy (H to He transition
liberates energy because He is more
stable) (strong nuclear force dominates)
(p n)
235U and 238U are isotopes each with 92 protons
and with 143 and 146 neutrons,
respectively. 235U is the only naturally
occurring fissionable isotope
26
Not all nuclei are stable unstable nuclei are
converted to stable by nuclear decay The decay
releases energy as radioactivity Types of decay
a-particle emission (He nucleus) b-particle
emission (electron)
27
(here A signifies the mass number p n, while Z
is the atomic number of protons)
28
Decay of 238U
Decay of heavy elements generally proceeds in a
sequential cascade Decay here is by
sequential a-particle and b-particle
emissions to form a stable lead
isotope Depending on the decay rates the
intermediates may build up 222Rn is on the
decay chain it is a noble gas and can diffuse
and build up inside homes located near
naturally occurring uranium dsposits
a
a
b
29
Nuclear decay process is always exponential,
though decay rates vary widely among
radioisotopes Rate of decay is proportional to
the number of nuclides in a sample Rate
-dN/dt kN (first order) ? ln (N/No) -kt and
t1/2 ln(2)/k