Typical Ion Exchange Capacities of Zeolites PowerPoint PPT Presentation
1 / 36
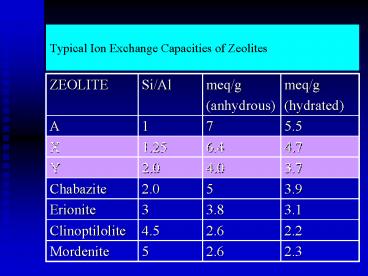
Title: Typical Ion Exchange Capacities of Zeolites
1
Typical Ion Exchange Capacities of Zeolites
2
Selectivity Series for Zeolite A
- Zeolite A
- AggtTlgtNagtKgtNH4gtRbgtLigtCs
- ZngtSrgtBagtCagtCogtNigtCdgtHggtMg
- Used in detergents for water softening (replacing
Ca2 and Mg2 with Na)
3
Ion-exchange isotherms for Li, K, Ag, and Ca
on zeolite NaA at 25 C Total concentration in
aqueous phase 0.1 N
4
Figure . Ion-exchange isotherms on zeolite X,
sodium form. X equivalent fraction in zeolite, X
equivalent fraction in solution.
5
Zeolites as Detergent Builders
- Detergent Builders The prime function of
phosphates in detergents is to reduce the
activity of the hardness ions, Ca2 and Mg2, in
the wash water by complexing. Zeolite ion
exchangers in powder form replace Ca2 and Mg2
in thes olution with ions such as Na. Heavy-duty
detergents employ the sodium form of Type A
zeolite for this purpose in low or zero-phosphate
formulations. The zeolite powder is incorporated
into the detergent powder during formulation.
Large amounts of zeolite are used in this
application.
6
SEM Micrographs of Clinoptilolite sample from
Gördes (MANISA)
7
Selectivity Sequence of Clinoptilolite( according
to different literature)
8
Uses of Clinoptilolite in Ion Exchange
- Removal of Ammonium ion (NH4) from wastewater
- Removal of radioactive 137Cs and 90Sr2 from
radioctive waste streams - Removal of heavy metals like Pb2 and
- Cd2 from wastewaters (potential )
9
- WHY REMOVAL OF AMMONIUM IS NEEDED ?
- ? toxic to fish aquatic life
- ? contribute to explosive algal growth
promoting eutrophication - ? dissolved oxygen reduction
- ? corrosive to certain metals materials of
construction - detrimental effect on disenfection of water
supplies - allowable concentration at 180 C and pH5-7 ? 2
mg/l
10
Zeolites in radioactive waste treatment
- Removal Cesium and Strontium Radioisotopes
- Because of their stability in the presence of
ionizing radiation and in aqueous solutions at
high temperatures, molecular sieve ion exchangers
offer significant advantages in the separation
and purification of radioisotopes. - Their low solubility over wide pH ranges,
together with their rigid frameworks and
dimensional stability and attrition resistance,
have endowed zeolites with properties which
generally surpass those of the other inorganic
and organic ion exchangers. - The high selectivities and capacities of several
zeolites for cesium and strontium radioisotopes
resulted in the development of processes
currently used by nuclear processing plants.
11
Equipment Types
- Batch
- Fixed bed
- Fluididized bed
- Continuous countercurrent
12
Batch Operations
Stirred tanks are used for batch contacting,
with an attached strainer or filter to remove the
resin beads from the solution after equilibrium
conditions are approached. Agitation is mild to
avoid resin attrition, but sufficient to achieve
complete suspension of the resin.
13
Fixed Bed Operation
- Semicontinuous and continuous systems are, with
few exceptions, practiced in columns. - Most columnar systems are semicontinuous since
flow of the stream being processed must be
interrupted for regeneration. - Columnar installations almost always involve the
process stream flowing down through a resin bed.
Those that are upflow use a flow rate that either
partially fluidizes the bed, or forms a packed
bed against an upper porous barrier or
distributor for process streams.
14
An industrial ion-exchange column a)
Distributor b) Resin c) Collector
15
Figure Examples of strainers used for industrial
water-softening plants A) Simple plate strainer
B) Double strainer for two-chamber floating
beds
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
Fixed Bed Cycle
- Sorption(loading)
- Backwash
- Regeneration
- Rinsing
20
Sorption(loading)
- Impurities are removed, or valuable constituents
recovered, from a process stream during the
adsorption step, which is also referred to as
loading or exhausting the resin. - Performance is rated primarily on meeting
objectives for completeness of removal. - Performance is also rated on operating capacity,
frequency of regeneration, and operational costs. - Variables affecting performance include resin
selection, solution chemistry, operating
conditions, and equipment design. All are
interrelated in varying degrees. - Completeness of removal improves by using a resin
more selective for that constituent. Using a
resin having a selectivity substantially greater
than required for the process stream generally
results in lower operating capacity, more
frequent regenerations, higher operating costs,
and higher capital investment. For example,
strong acid rather than weak acid cation
exchangers are used to soften water supplies
21
Operating Capacity in fixed bed operation
- is defined as proportion of total(equilibrium)
capacity used during the exchange process,
depends on - Concentration and types of ions to be sorbed
- Rate of percolation
- Temperature
- Depth of resin bed
- Type, concentration and quantity of regenerant
22
(No Transcript)
23
Backwash
- Process streams may contain small suspended
particles which are collected on top of the
resin bed, and penetrate deeper down causing an
increase pressure drop across the bed. - Water is passed up through a bottom distributor
at a flow rate sufficient to expand the resin bed
by 50100, and exits the top of the column No
resin, other than a small amount that may have
undergone physical degradation, should escape the
unit as long as the column was designed to
accommodate that degree of expansion . Tap water
may be used as backwash. Backwash frequency
varies from one installation to another
24
Regeneration
- The regeneration step is also called elution..
- Regeneration is of much shorter duration than the
adsorption step. - The combined time for backwashing, regeneration,
and rinse is usually not longer than two hours.
The time is shortened using a smaller volume of
regenerating chemicals at a higher concentration,
or by increasing the regenerant flow rate. - Flow rates commonly used for regeneration in
terms of bed volumes (BV) are between 4 6 BV/h,
but 2 BV/h is preferred in many cases
25
- Cation exchangers are regenerated with mineral
acids when used in the H form. - Sulfuric acid is preferred over hydrochloric
acid , HCl, in many countries because it is less
expensive and less corrosive. - However, the use of hydrochloric acid is the
best method of overcoming precipitation problems
in installations which deionize water with high
concentrations of barium or calcium compared to
other cations. - A 4 acid concentration is common, although
sulfuric acid regenerations may start as low as
0.81 to minimize calcium sulfate precipitation.
26
- Strong base anion exchangers must be regenerated
with sodium hydroxide when used in the OH- form. - Potassium hydroxide is a more expensive
alternative. - Weak base anion exchangers may be regenerated
with solutions of ammonium hydroxide , NH4OH, or
sodium carbonate , Na2CO3, although NaOH is more
common. The most common concentration for basic
regenerating solutions is 4.
27
Co-flow and coounterflow regeneration
28
- Mixed-bed resins cannot be regenerated until the
two resins are separated by backwashing. Each
resin is regenerated separately. The cation
exchanger should not be in contact with the NaOH
solution used for the anion exchanger. The anion
exchanger should not be in contact with the acid
solution used to regenerate the cation exchanger.
29
Rinsing
- When transfer of the required volume of
regenerating solution to the column has been
completed, a small amount of regenerating
solution occupies space immediately above the
resin bed, between resin particles in the bed,
and within the resin particles. - It must be displaced with water before the column
can be returned to the adsorption step. - Rinsing should begin at the same flow rate as
used during regeneration and continue at that
rate until a volume of water equal to 12 bed
volumes has been used. After that, the flow rate
is increased to the rate normally used during the
adsorption step, and continued at that rate until
the effluent is of satisfactory quality, as
determined by pH, conductivity, or resistivity. .
30
(No Transcript)
31
The Himsley contactor has a series of trays, on
each of which the resin beads are fluidised by
the upward flow of liquid. Periodically, the flow
is reversed to move incremental amounts of resin
from one stage to the stage below. The batch of
resin at the bottom is lifted to the wash column,
then to the regeneration column, and then back to
the top of the ion exchanger column for re-use.
32
Higgins contactor operates as a moving, packed
bed by using intermittent hydraulic pulses to
move incremental portions of the bed from the
contacting section, where ion exchange takes
place, up around and down to the backwash
section, down to the regeneration section, and
back up through the rinse section to the
contacting section to repeat the cycle. Liquid
moves counter-currently to the resin
33
Mixed Bed Operations
34
Mixed Bed Ion Exchange
35
Figure Mixed-bed ion-exchange system a)
Breather b) Raw water distributor c) Sodium
hydroxide distributor d) Intermediate collector
e) Strainer rack
36
Figure . Small-scale water softener a) Control
unit b) Resin c) Solid salt d) Saturated brine