Students - PowerPoint PPT Presentation
Title:
Students
Description:
... property of entropy are significantly different when problems are posed using ... Consider one complete cycle; the system begins in a certain state and returns to ... – PowerPoint PPT presentation
Number of Views:32
Avg rating:3.0/5.0
Title: Students
1
Students Ideas About the State-Function Property
of Entropy Warren M. Christensen, David E.
Meltzer, Thomas A. Stroman Iowa State
University Supported in part by NSF grants
DUE-9981140 and PHY-0406724
Previous Results
A fundamental concept of thermodynamics is that a
system in a particular state has a set of
properties that is unique to that state. When a
system changes from some initial state to some
final state, the change of a given state function
is the same regardless of how the system gets
from the initial to the final state. Heat
transfer, Q, is not a state function and its
value depends on the process that the system
undergoes. Student thinking regarding these
quantities have been studied by Loverude, et al.,
AJP, 2002 and Meltzer, AJP, 2004 in the
context of the first law of thermodynamics.
Which would produce the largest change in the
total energy of all the atoms in the system
Process 1, Process 2, or both processes produce
the same change?
In 2001, 73 of students taking a second-semester
calculus-based physics course (N 279)
determined correctly that the change in total
energy would be the same for both processes.
Is Q for Process 1 greater than, less than, or
equal to that for Process 2?
1999 2000 2001
Incorrect N 186 N 188 N 279
Q1 Q2 31 43 41
Heat transfer is not a state function but 40 of
students give answers consistent with that idea.
From Meltzer 2004
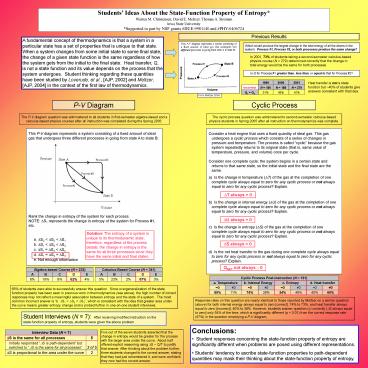
P-V Diagram
Cyclic Process
The P-V diagram question was administered to all
students in first-semester algebra-based and a
calculus-based physics courses after all
instruction was completed during the Spring 2005.
The cyclic process question was administered to
second-semester calculus-based physics students
in Spring 2005 after all instruction on
thermodynamics was complete.
- Consider a heat engine that uses a fixed quantity
of ideal gas. This gas undergoes a cyclic process
which consists of a series of changes in pressure
and temperature. The process is called cyclic
because the gas system repeatedly returns to its
original state (that is, same value of
temperature, pressure, and volume) once per
cycle. - Consider one complete cycle the system begins in
a certain state and returns to that same state,
so the initial state and the final state are the
same. - Is the change in temperature (?T) of the gas at
the completion of one complete cycle always equal
to zero for any cyclic process or not always
equal to zero for any cyclic process? Explain. - Is the change in internal energy (?U) of the
gas at the completion of one complete cycle
always equal to zero for any cyclic process or
not always equal to zero for any cyclic process?
Explain. - Is the change in entropy (?S) of the gas at the
completion of one complete cycle always equal to
zero for any cyclic process or not always equal
to zero for any cyclic process? Explain. - Is the net heat transfer to the gas during one
complete cycle always equal to zero for any
cyclic process or not always equal to zero for
any cyclic process? Explain.
DT always 0
DU always 0
DS always 0
QNET not always 0
Calculus-Based Course (N 341) Calculus-Based Course (N 341) Calculus-Based Course (N 341) Calculus-Based Course (N 341) Calculus-Based Course (N 341)
A B C D E
5 23 2 67 3
Algebra-based Course (N 232) Algebra-based Course (N 232) Algebra-based Course (N 232) Algebra-based Course (N 232) Algebra-based Course (N 232)
A B C D E
6 19 9 62 4
Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191) Cyclic Process Post-Instruction (N 191)
a. Temperature a. Temperature b. Internal Energy b. Internal Energy c. Entropy c. Entropy d. Heat transfer d. Heat transfer
0 ?0 0 ?0 0 ?0 0 ?0
89 11 74 26 54 46 40 60
65 of students were able to successfully answer
this question. Since overgeneralization of the
state function property has been seen in previous
work in thermodynamics (see above), the high
number of correct responses may not reflect a
meaningful association between entropy and the
state of a system. The most common incorrect
answer is b. DS1 lt DS2 lt DS3, which is
consistent with the idea that greater area under
the curve means greater entropy change and is
probed further in one-on-one student interviews.
Response rates on this question are nearly
identical to those reported by Meltzer on a
similar question (above) for both internal energy
always equal to zero correct, 74 to 73, and
heat transfer always equal to zero incorrect,
40 to 38. However, students answer question (c)
correctly DS always equal to zero only 54 of
the time, which is significantly different (p lt
0.01) from the correct response rate (67) in the
question employing a P-V diagram.
Student Interviews (N 7) After receiving
modified instruction on the state-function
property of entropy, students were given the
above problem.
- Conclusions
- Student responses concerning the state-function
property of entropy are significantly different
when problems are posed using different
representations. - Students tendency to ascribe state-function
properties to path-dependent quantities may mask
their thinking about the state-function property
of entropy.
Interview Data (N 7) Interview Data (N 7)
DS is the same for all processes 5
Initially responded DS is path-dependent but switched to DS is the same for all processes 3 of 5
DS is proportional to the area under the curve 2
Five out of the seven students asserted that the
change in entropy would be greater for the
process with the larger area under the curve.
About half offered explicit reasoning using DS
Q/T to justify that answer. After thinking about
the problem further, three students changed to
the correct answer, stating that they had just
remembered it, and were confident they now had
the correct answer.