Goals: PowerPoint PPT Presentation
1 / 18
Title: Goals:
1
Chapter 12 Infrared Spectroscopy and Mass
Spectrometry
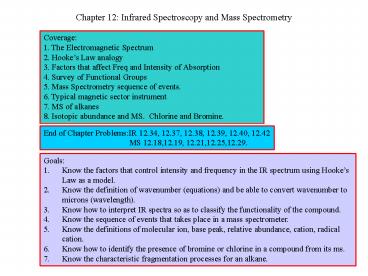
Coverage 1. The Electromagnetic Spectrum 2.
Hookes Law analogy 3. Factors that affect Freq
and Intensity of Absorption 4. Survey of
Functional Groups 5. Mass Spectrometry sequence
of events. 6. Typical magnetic sector instrument
7. MS of alkanes 8. Isotopic abundance and MS.
Chlorine and Bromine.
End of Chapter ProblemsIR 12.34, 12.37, 12.38,
12.39, 12.40, 12.42
MS 12.18,12.19, 12.21,12.25,12.29.
- Goals
- Know the factors that control intensity and
frequency in the IR spectrum using Hookes Law as
a model. - Know the definition of wavenumber (equations) and
be able to convert wavenumber to microns
(wavelength). - Know how to interpret IR spectra so as to
classify the functionality of the compound. - Know the sequence of events that takes place in a
mass spectrometer. - Know the definitions of molecular ion, base peak,
relative abundance, cation, radical cation. - Know how to identify the presence of bromine or
chlorine in a compound from its ms. - Know the characteristic fragmentation processes
for an alkane.
2
(No Transcript)
3
Infrared Spectroscopy (IR)
IR spectroscopy measures the amount of infrared
light absorbed by a molecule as a function of the
frequency of the light.
Basic relationships E h? where h is Plancks
constant, ? is frequency in cycles/s or Hz E
hc where c is speed of light (3 x 1010 cm/sec),
? is wavelength of light in cm ?
?
4
The absorption of IR light by a molecule results
in changes in the vibrational energy level of the
molecule. Analogy Two masses connected by a
spring, vibrating at a frequency ?
m2
m1
where mreduced m1m2__
m1 m2
?? ? k/mreduced
k is the force constant of the spring and is
related to the strength of the bond. Strong bond
? large k ? high ? mreduced is the reduced mass
of the bonded atoms Large mreduced, low ?
5
If the frequency of the IR light matches the
frequency of the molecular vibration, light will
be absorbed by the molecule and the amplitute of
the vibration will increase.
IR spectrum of 1-butanol, CH3CH2CH2CH2OH
T
4000
500
Wavenumber, ?, cm-1
O-H stretch
C-H stretch
C-H bend C-O stretch
6
Frequency of Absorption depends on a. strength
of bond (k force constant)
Stronger bond, absorbs at higher ?, 2250 cm-1
Intermediate, absorbs at 1660 cm-1
Weaker bond, absorbs at lower ? , 1200 cm-1
b. Reduced mass of the atoms
H is lighter atom, absorbs at higher ?, 3000 cm-1
D is heavier atom, absorbs at lower ?, 2100 cm-1
7
- Intensity of Absorption depends on
- The polarity of the bond. The more polar, the
more intense the peak. - example CO very polar, intense
peak - C?C nearly
nonpolar, weak peak
- The concentration of the molecule or the number
of bonds. Absorption - follows Beers Law, that is, Absorbance
? concentration
Alkane octane
4000 3000 2000
1500 1000
500
Csp3-H stretch 2900 Csp3-H bend 1450,
1375
8
Alkenes 1-octene
4000 3000 2000
1500 1000
500
Csp2-H stretch 3080 CC stretch 1640
Alkynes 1-octyne
4000 3000 2000
1500 1000
500
?C-H stretch 3315 C?C stretch 2120
9
Alcohols 1-butanol
T
4000 3000 2000
1500 1000
500
O-H stretch 3300 (broad)
C-O stretch 1050
Amines butylamine
4000 3000 2000
1500 1000
500
N-H stretch 3370,3290 N-H bend,
1606
10
Ketones 2-pentanone
4000 3000 2000
1500 1000
500
CO stretch 1717
Aldehydes pentanal
4000 3000 2000
1500 1000
500
CO stretch 1727
OC-H 2720
11
Carboxylic Acid Benzoic acid
(solid)
KBr pellet
4000 3000 2000
1500 1000
500
O-H stretch 2500-3200 CO stretch 1689
12
Mass Spectrometry (MS)
MS provides molecular weight of the molecule.
Fragmentation of the molecule also Occurs. Mass
of the fragments give insight into structure.
- Sequence of events inside a mass spectrometer
- Vaporization of the molecule in high vacuum.
- Ionization by Electron-impact, i.e. Electrons
are shot at the molecule and - causes it to ionize.
radical cation
e- CH4 ? 2e- CH4
- Fragmentation of original radical cation
CH4 ? CH3 H
4. Acceleration 5. Separation of ions into
different masses.
13
Diagram of a Magnetic Sector Mass Spectrometer
14
The radius of curvature of the ion depends on the
mass/charge ratio (m/z). Large mass ? large
radius Small mass ? small radius
By varying the strength of the magnetic field,
the spectrometer scans all possible ion masses
and produces a graph of the number of ions of
each mass.
6. Detection. The ions are counted and their
mass is measured to produce the mass
spectrum.
15
Mass Spectrum of Octane
Base peak
43
114
Molecular ion, M
Base peak peak corresponding to the most
abundant ion, defined as 100
relative abundance. Molecular ion (M) ion
corresponding to molecular weight of compound.
This peak is often
not the most abundant ion
16
What are the fragments of this molecule?
43
57
29
85
71
29 43 57 71 85
C6H13
C2H5
C5H11
C3H7
C4H9
17
Some other recognizable patterns in MS
1-bromobutane
79Br 50.5 81Br 49.5
CH3CH2CH2CH279Br
CH3CH2CH2CH281Br
M 136
M2 138
There are two isotopes of bromine in nature, 79Br
and 81Br, in roughly equal abundance.
Therefore there are two peaks of equal intensity
at m/z of 136 and 138, corresponding to the
molecular ions but with different Isotopes.
18
Chlorobenzene
35Cl 75.5 37Cl 24.5
77
M2 114
M 112
There are two isotopes of chlorine in nature,
35Cl and 37Cl, in roughly a 31 ratio. Therefore
there are two peaks of equal intensity at m/z of
112 and 114 in a 31 ratio of intensities,
corresponding to the molecular ions but with
different isotopes.