Equilibrium and noequilibrium processes' - PowerPoint PPT Presentation
1 / 33
Title:
Equilibrium and noequilibrium processes'
Description:
Connection between statistical and thermodynamic quantities. Helmholtz free energy F, Enthalpy ... Recapitulation of thermodynamic laws. Zero law. First law- 15 ... – PowerPoint PPT presentation
Number of Views:64
Avg rating:3.0/5.0
Title: Equilibrium and noequilibrium processes'
1
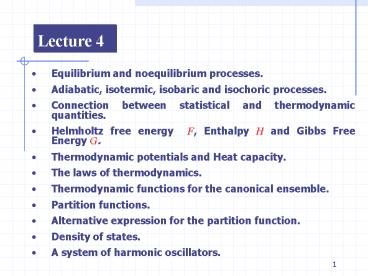
Lecture 4
- Equilibrium and noequilibrium processes.
- Adiabatic, isotermic, isobaric and isochoric
processes. - Connection between statistical and thermodynamic
quantities. - Helmholtz free energy F, Enthalpy H and Gibbs
Free Energy G. - Thermodynamic potentials and Heat capacity.
- The laws of thermodynamics.
- Thermodynamic functions for the canonical
ensemble. - Partition functions.
- Alternative expression for the partition
function. - Density of states.
- A system of harmonic oscillators.
2
Equilibrium and noequilibrium process
The typical example of nonreversible ( in
statistical sense) process is the relaxation
process.
The process is reversible if during every its
moment the system is in equilibrium state and the
process can go any direction. The reversible
processes are usually connected with some
variations of external conditions and the energy
of the system.
The variations have to be so slow that the system
can reach equilibrium. Very slow process can be
defined as quasystatic. The meanings slow
depends on process time and have to be compared
with the relaxation time.
Adiabatic processes can be defined as the
processes at the constant material and
temperature conditions. Isothermal, isobar and
isochoric processes are going at a constant
temperature, pressure and volume respectively
3
Connection between Statistical and Thermodynamic
Quantities
We have seen that for a system in equilibrium
??(E,x?,Ni), where E is the energy x? denote
the set of external parameters describing the
system and the Ni are the numbers of molecules
of the several chemical species present. If the
conditions are changed slightly, but reversibly
in such a way that the resulting system is also
in equilibrium, we have
(4.1)
4
We may write this result as
(4.2)
Let us first consider a simple example with the
number of particles fixed and the volume as the
only external parameter
(4.3)
Then from (4.2)
(4.4)
We see that the change in internal energy
consists from two parts. The term ?d? represents
the change in internal energy when the external
parameters are kept constant. This is just what
is meant by heat. Thus
5
(4.5)
is the quantity of heat added to the system in a
reversible process. The symbol D is used instead
of d because DQ is not an exact differential-that
is, Q is not a state function.
The term -? dV is the change in internal energy
caused by the change in external parameters this
is what we mean by mechanical work, and
(4.6)
is the work done on the system in the volume
change dV.
By elementary mechanics the work done must be
given by -pdV. Therefore
(4.7)
6
where p is the pressure. We see that (4.2) is
equivalent to the equation
(4.8)
which is the First Law of Thermodynamics.
The statement that dSDQ/T is a perfect (exact)
differential in a reversible process is a
statement of the Second Law of Thermodynamics.
That is, DQ/T is a differential of state
function, entirely defined by the state of the
system. Now from (4.5) we know that
7
Specific Heat
(4.9)
is a perfect differential, as ? is a state
function. We note that both 1/T and 1/? are
integrating factors for DQ. As we know that ?kT,
thus
(4.10)
as the connection between the usual thermodynamic
entropy S and the entropy ? as we prefer to
define it for use in statistical mechanics.
The specific heat at constant volume Cv, and the
one at constant pressure, Cp, would be given by
8
We defined E as a function of ? and V. Other
quantities of interest are then obtained from E
(4.11)
whence
(4.12)
and
(4.13)
The independent variables ? and V are often quite
inconvenient and it is more convenient to work
with ?, p or ?, V for example.
9
Helmholtz Free Energy F
To do this we introduce auxiliary functions
called thermodynamic potentials F, H, G.
F(V, ?) is defined as
(4.14)
Now
(4.15)
From (4.15)
(4.16)
and
(4.17)
10
Enthalpy H
Therefore if V, ? are the independent variables
it is natural to introduce F, from which p,? are
readily calculated
H(?,p) is defined by
(4.18)
Now
(4.19)
whence
(4.20)
and
(4.21)
11
Gibbs Free Energy G
G(?,p) is defined by
(4.22)
Now
(4.23)
whence
(4.24)
and
(4.25)
12
The Helmholtz free energy of a body has the
property that the work done on the body in a
reversible process at constant temperature is the
change of its Helmholtz free energy.
This easily shown in a reversible process
(4.26)
Note that -dF is the maximum work, which can be,
done by the system in a change at constant
temperature.
In the case of one component system with the
volume as only one external parameter we can
write the main thermodynamic equation for
quasi-static processes
(4.27)
13
Thermodynamic potentials
14
Recapitulation of thermodynamic laws
-postulated the existence of
equilibrium states. All parts of closed
equilibrium system are in the state of internal
equilibrium and heat equilibrium between each
other, that means one general characteristic
from all subsystems is taking place (temperature
principle).
Zero law
the law of energy continuity.
The energy can be transformed to the system by
the heat. It impossible to make any work without
the energy. (The perpetual mobile of the 1st
order is impossible).
First law-
15
the entropy of the close
system is increasing. It can be defined also
through the Clausius principle as the
irreversible process of the transforming the heat
from the hot body to the cold one.
Second law-
As the principle of Kelvin (Tomson) the second
law read It is impossible to build the cycle
machine that can work by absorption of the heat
from the thermostat with any other changes in the
system (the perpetual mobile of the 2nd order can
not be created).
(Nernst-Plank heat
theorem) - the entropy of the system is going to
zero if the absolute temperature is also tends to
zero.
Third Law -
16
Thermodynamic Functions for the Canonical Ensemble
Let us define the entropy of the canonical
ensemble with mean energy ltEgt as being equal to
the entropy of a microcanonical ensemble with
energy ltEgt.
This corresponds to the thermodynamic situation
because in thermodynamics the entropy is fixed by
the energy independently of whether the system is
isolated or in contact with a heat bath.
The entropy for the microcanonical ensemble is
equal to ln?? where ?? is the volume of phase
space corresponding to energies between E0 and
E0?E. As we have seen, the precise value of ?E
is unimportant and we may choose it equal to the
range of reasonable probable values of the energy
in the canonical ensemble.
17
Let us first write ?? in terms of ?E. If ?(E)
denotes the volume of phase space corresponding
to energies less than E we have
(4.28)
We now estimate ?E, the range of reasonable
probable values for the canonical ensemble. Let
p(E)dE be the canonical ensemble probability that
the system will have energy in the range dE at
E. Then,
(4.29)
where ?(E) is the occupancy probability of a unit
volume of phase space at energy E. p(E) is
distributed according to the Gauss distribution.
The function is normalized and this means that we
may estimate the breadth ?E of the distribution
peak by
18
(4.30)
i.e., by
(4.31)
Substituting the ?E given by this equation in the
expression for ??, we obtain, using (3.39)
(4.32)
so that
(4.33)
We have
19
(4.34)
But we recall the Helmoholtz free energy F?E-??,
whence
(4.35)
and
(4.36)
We have further, by the normalization of ?
(4.37)
and
(4.38)
20
The partition function
If we define the partition function as
(4.39)
(classical)
(quantum)
(4.40)
we have
(4.41)
The other thermodynamic functions can be
calculated from the partition function, using
thermodynamic potentials.
21
Alternative expression for the partition
function. Density of states.
In most physical cases the energy levels
accessible to a system are degenerate, i.e. one
has a group of states, gr in number, all
belonging to the same energy value Er . In such
a case it would be more appropriate to write the
partition function
(4.42)
the corresponding expression for Pr , the
probability that the system be in any of the
states with energy Er , would be
22
(4.43)
Clearly, the gr states with a common energy Er
are all equally likely to occur. As a result the
probability of a system having energy Er becomes
directly proportional to the multiplicity gr of
this level gr thus plays the role of "weight
factor" for the level Er. The actual probability
is then determined by both the weight factor gr
and the Boltzmann factor exp(-Er/kT) of the
level, as we indeed have in (4.43).
Now in view of the largess of the number of
particles constituting a given system and the
largess of the volume to which these particles
are confined, the consecutive energy values Er of
the system must be extremely close to one
another.
23
Accordingly, there lie, within any reasonable
interval of energy (E,EdE), a very large number
of energy levels.
One may then regard E as a continues variable and
write P(E)dE for the probability that the given
system, as a member of the canonical ensemble,
may have its energy in the specified range.
Clearly, the product of the relevant single-state
probability and the number of energy states lying
in the specified range will give this. Denoting
the latter by g(E)dE, where g(E) stands for the
density of states of the system around the energy
value E, we have
(4.44)
24
which on normalization becomes
(4.45)
The denominator is clearly another expression for
the partition function of the system
(4.46)
The expression for ltfgt any average value of
physical quantity f may be written in this case as
25
(4.47)
Let us consider the relation (4.46) If we regard
?1/kT as a complex variable, then the partition
function Z(?) is just Laplace transform of the
density of states g(E).
We can, therefore, write g(E) as the inverse
Laplace transform of Z(?)
26
(4.48)
the path of integration runs parallel to, and to
the right of, the imaginary axis, i.e. along the
straight line Re ??? gt0.
27
A system of harmonic oscillators
We shall now study, as an example, a system of N,
practically independent, harmonic oscillators. We
start with the specialized situation when the
oscillators can be treated classically. The
Hamiltonian of any one of them (assumed to be
one-dimensional) may then be written as
(4.49)
of course, the index i will run from 1 to N. For
the single-oscillator partition function, we
readily obtain
(4.50)
28
where
The partition function of the N-oscillator system
would then be
(4.51)
The Helmholtz free energy of the system is now
given by
(4.52)
whence we obtain for other thermodynamic
quantities
(4.53)
(4.54)
(4.55)
(4.56)
(4.57)
29
We note that the mean energy per oscillator is in
complete accord with the equipartition theorem,
namely 2? , for E we have here two
independent quadratic terms in the single
oscillator Hamiltonian.
We may determine the density of states, g(E), of
this system from the expression (4.51) for its
partition function. We have, in view of (4.48),
(?'gt0),
that is
(4.58)
30
To test this correctness, we may calculate the
entropy of the system with the help of this
formula. Taking Ngtgt1 and making use the Stirling
approximation, we get
(4.59)
which yields for the temperature of the system
(4.60)
Eliminating E between these two relations, we
obtain precisely our earlier result for the
functions S(N,T). This indeed assure us of the
inner consistency of our approach more so, it
gives us confidence to accept (4.58) as the
correct expression for the density of states of
this system.
31
We now take up the quantum-mechanical situation,
according to which the energy eigenvalues of a
one-dimensional harmonic oscillator are given by
(4.61)
n0,1,2,...
Accordingly, we have for the single-oscillator
partition function
(4.62)
The N-oscillator partition function is then given
by
(4.63)
32
For the Helmholtz free energy of the system, we
have
(4.64)
whence we obtain for other thermodynamic
quantities
(4.65)
(4.66)
(4.67)
(4.68)
33
(4.69)