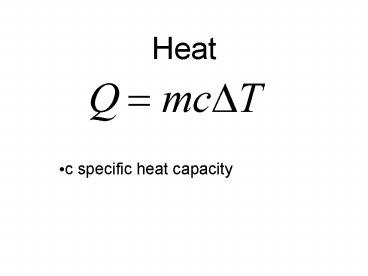Heat - PowerPoint PPT Presentation
1 / 23
Title: Heat
1
Heat
- c specific heat capacity
2
Specific heat capacity of Liquid water
- Liquid water has a high specific heat capacity
3
Specific heat capacity
- Wood coals have a low specific heat capacity
- Ash is a poor conductor of heat
- Keep moving
4
Specific heat capacity
5
Heat of fusion
- Amount of energy required to melt or freeze a
substance
6
Heat of vaporization
- The amount of heat required to boil a liquid that
is already at the boiling temperature
7
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
Step 1
8
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
melt
0C
0C
Step 2
- Notice that the heat only melted the ice and that
the temperature remains constant
9
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
warm
Step 3
0C
100 C
- Use this warming equation, every time there is
a temperature change
10
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
Step 4
Boil
100C
100C
- Notice that in this step, the heat only changes
the state of the water, not its temperature
11
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
Step 5
warm
- Another place where the temperature does change
12
How much heat is needed in order to change the
temperature of a 10 gram ice cube that is at
-30C, to a vapor at 140C?
150 calories 800 calories 1000 calories
5400 calories 200 calories 7550 calories
Step 6
- The total energy required will be 7,550 calories
13
The energy that powers storms, comes from the
sun. The energy is stored in water when it
evaporates from the ocean.
Katrina 2005
14
Why is it dangerous to open the radiator cap when
the engine is hot?
- The water in the cars cooling system is
pressurized. Its temperature is well above the
normal boil point of water. - When the cap is opened, the pressure is reduced
and much of the water vaporizes-causing an
explosion.
15
1st law of thermodynamics A conservation of
energy statement
- ?U change in internal energy
- W work
- Q heat
16
1st law of thermodynamics A conservation of
energy statement
- If heat flows into a system, and no work is done,
its internal energy must increase. - If heat flow out of a system, and no work is
done, its internal energy must decrease.
17
1st law of thermodynamics A conservation of
energy statement
- If the gas does work, it must lose energy.
18
How does a gas do work?
- As it expands, it pushes surrounding air away.
- Work done an the expanding gas, equals its
pressure times the change in its volume.
19
What does or - W mean?
- If work is done to a gas, its internal energy
must increase. It must gain energy. - - If work is done by the gas, its internal energy
must decrease. It must lose energy.
20
Adiabatic cooing
- When a warm air mass rises, it will be surrounded
by air of less pressure. Therefore the rising
air expands, does work and cools down.
21
Adiabatic warming
- As a cold air mass sinks, it is surrounded by air
of higher pressure. The sinking air mass is
compressed-work is done to it. - The sinking air mass gains energy and warms up.
22
The secret behind refrigeration
- A gas is pressurized into a liquid by a
refrigerator motor-compressor. It warms up and
is radiated away into the kitchen. - The liquid is then sent to a larger diameter tube
and expands into a gas. - The expanding gas does work and must therefore
lose energy. - The cold gas is in the tubes that are in the
refrigeration unit. - The gas goes back to the compressor and the cycle
starts again.
23
The second law of thermodynamics
- Left to itself, the natural tendency of a system
is for its entropy to increase. - Entropy is a measure of a systems disorder.