Interfaces - PowerPoint PPT Presentation
1 / 45
Title:
Interfaces
Description:
Surfactants are molecules that preferentially adsorb at an. interface, i.e. ... Adsorb within micelle and reduce CMC. Typically polar. molecules, e.g. alcohols ... – PowerPoint PPT presentation
Number of Views:126
Avg rating:3.0/5.0
Title: Interfaces
1
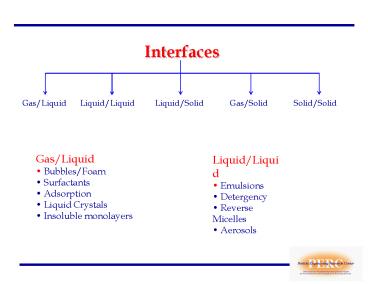
Interfaces
Gas/Liquid
Liquid/Liquid
Liquid/Solid
Gas/Solid
Solid/Solid
- Gas/Liquid
- Bubbles/Foam
- Surfactants
- Adsorption
- Liquid Crystals
- Insoluble monolayers
- Liquid/Liquid
- Emulsions
- Detergency
- Reverse Micelles
- Aerosols
2
Adhesion of Surfaces
- Work of COHESION, W11
- Energy needed to separate two identical
- surfaces from contact to infinite separation
- Work of ADHESION, W12
- Energy needed to separate two dissimilar
- surfaces from contact to infinite separation
- Units of energy per unit area (mJ/m2 erg/cm2)
3
Surface Free Energy
- Process of creating new surface area equivalent
to separating two half spaces. - Surface Energy (Solid/Gas)
- For Liquid/Liquid Interface, usually termed
Interfacial Tension - For Gas/Liquid interface usually termed
Surface Tension - with units of force/unit length (mN/m)
- note mN/m mJ/m2
4
Surface Tension
- Imbalance of forces at the Gas/Liquid Interface
- Surface tension (g) decreases with increasing
temperature - g decreases with increasing pressure
5
Contact Angle, q (Young-Laplace Equation)
gLG
?
gSG
gSL
At equilibrium all tensions must be balanced
gSG gSL gLGcosq For a
water droplet on a surface 0 lt q lt 90
hydrophilic , 90 lt q lt 180, hydrophobic
TiO2-Silicone film after UV irradiation
TiO2-Silicone film before UV irradiation
6
Determination of Surface Energy (Zisman Plot)
Contact angle measurement of a solid with
various liquids of known surface tension
Cos q 1 b(gl - gc)
Cos q
q Contact angle b 0.03 0.04 gl Surface
tension of liquid gc Critical surface tension
g1
Extrapolate curve to Cos q 1, obtain ?c ,
characteristic of Surface Energy
(Adamson, 1997)
7
Young-Laplace Equation Equation of Capillarity
- - Interfacial Tension
- P - Pressure
- R - Radius
- Pressure inside a drop or bubble is always
greater than in the continuous phase. - The balance between surface tension and external
forces (e.g. gravity) dictate the shape of drops
and bubbles.
8
Surface Tension Measurement -- du Nouy ring --
Adamson, Physical Chemistry of Surfaces, 2nd Ed
p. 22 (1976)
wttotal total weight wtring ring weight R
ring radius g surface tension
- Still commonly used but values may be as much as
25 in error.
9
Surface Tension Measurement -- Wilhelmy Plate --
g surface tension q contact angle wttotal
total weight
wtplate plate weight b buoyancy force l
width of plate
- Normally platinum is used to have q ? 0 and
plate just touches liquid so buoyancy is small
10
Surface Tension Measurement -- Drop Weight Method
--
Adamson, Physical Chemistry of Surfaces, 2nd Ed
p. 19 (1976)
W 2prg
W weight of droplet r radius of droplet g
surface tension
11
SURFACTANTS
12
BASIC TERMINOLOGY
- Hydrophilic A liquid/surface that has a high
affinity to water. - Hydrophobic A liquid/surface that has very low
affinity to water - Lipophilic A liquid/surface that has a high
affinity to oil. - Lipophobic A liquid/surface that has a very low
affinity to oil.
13
BASIC TERMINOLOGY
14
INTRODUCTION
- Surfactants are molecules that preferentially
adsorb at an - interface, i.e. solid/liquid (froth flotation),
liquid/gas - (foams), liquid/liquid (emulsions).
- Significantly alter interfacial free energy
(work needed to - create or expand interface/unit area).
- Surface free energy of interface minimized by
reducing - interfacial area.
15
SURFACTANT STRUCTURE
- Surfactants have amphipathic structure
- Tail or hydrophobic group
- Little affinity for bulk solvent. Usually
hydrocarbon (alkyl/aryl) chain in aqueous
solvents. Can be linear or branched. - Head or hydrophilic group
- Strong affinity for bulk solvent. Can be neutral
or charged.
16
SURFACTANT CLASSES
- Carboxylic acids and their salts including
various fatty acids tall oil acids, and
hydrolyzed proteins
- Sulfonic acids and their salts, including
hydrocarbon backbones of alkylbenzene, benzene,
naphthalene, toluene, phenolm lingin, olefins,
diphenyloxide, petroleum cuts, succinate esters
etc.
- Sulfuric acid or salts including sulfated
primary alcohols, sulfated polyxyalkylenated
alcohols etc.
- Alkyl xanthic acids
- Alkyl or aryl dithiophosporic acids
- Polymeric anionics involving repeated groups
containing carboxyl acid functionality
Anionic ( 60 of industrial surfactants)
17
SURFACTANT CLASSES (contd.)
- Long chain amines derived from animal and
vegetable acids, tall oil and synthetic amines
- Diamines and polyamines including ether amines
and imidazolines
- Quaternary ammonium salts including tertiary
mines and imidazolines
- Quaternized and unquartenized polyoxyalkylenated
long chain amines
- Amine oxides derived from tertiary amines
oxidized with hydrogen peroxide
Cationic ( 10 of industrial surfactants)
18
SURFACTANT CLASSES (contd.)
- Polyoxyethylenated alcohols, alkyl phenols,
alcohol ethoxylates including derivatives from
nonyl phenol, coconut oil, tallow, and synthetic
alcohols
- Polyoxyethylenated glycols
- Polyoxypropylenated glycols
- Esters of carboxylic acids and alkyene oxides
- Alkanolamine condensates with carboxylic acids
- Polyoxyalkylenated mercaptans
Non-ionic ( 25 of industrial surfactants)
19
SURFACTANT CLASSES (contd.)
- Acrylic acid derivatives with amine
functionality
- Subsituted alkylamides
- n-Alkyl betaines
- n-Alkyl suffobetaine
- Thio alkyl amines and amides
Amphoteric or zwitterionic ( 10 of industrial
surfactants). Generally expensive specialty
chemicals.
20
HYDROPHILIC-LIPOPHILIC BALANCE
- Griffin (1949) the hydrophilic-lipophilic
balance (HLB) of a surfactant reflects its
partitioning behavior between a polar (water) and
non-polar (oil) medium. - HLB number, ranging from 0-40, can be assigned
to a surfactant, based on emulsification data.
Semi-empirical only. - Strongly hydrophilic surfactant, HLB ? 40
- Strongly lipophilic surfactant, HLB ? 1
- HLB dependent upon characteristics of polar and
non-polar groups, e.g. alkyl chain length,
headgroup structure (charge, polarity, pKa).
21
HYDROPHILIC-LIPOPHILIC BALANCE -- Effect of
Structure --
22
HYDROPHILIC-LIPOPHILIC BALANCE
A value of 10 represents a mid-point of HLB.
23
HYDROPHILIC-LIPOPHILIC BALANCE
Translucent to clear solution
No dispersibility in water
Milky dispersion unstable
poor dispersibility in water
Clear solution
0
2
6
8
10
12
14
16
18
4
HLB
Water in oil emulsifier
Wetting agent
Detergent
Solubilizer
Insecticidal sprays
triglycerol monooleate Cream and ointment
stabilizers
Oil-in-water emulsifier
Polysorbate 20
24
MICELLES
- If concentration is sufficiently high,
surfactants can form - aggregates in aqueous solution ?
micelles. - Typically spheroidal particles of 2.5-6 nm
diameter.
Hartley Spherical Micelle McBain Lamellar
Micelle
Oil in Water Micelle Water in Oil Micelle
Surfactant Micelle
(Klimpel, Intro to ChemicalsUsed in Particle
Systems,p. 29, 1997, Fig 21)
25
MICELLES --CMC--
- Onset of micellization observed by sudden change
in - measured properties of solution at
characteristic surfactant - concentration
- ? critical micelle concentration
(CMC).
(Klimpel, Intro to ChemicalsUsed in Particle
Systems, p. 29, 1997, Fig 20)
26
MICELLES --CMC Trends--
- For the same head group, CMC decreases with
increasing alkyl chain length. - (2) CMC of neutral surfactants lower than ionic
- (2) CMC of ionic surfactants decreases with
increasing salt concentration. - (3) For the same head group and alkyl chain
length, CMC increases with increase in number of
ethylene oxide groups. - (4) For mixed anionic-cationic surfactants, CMC
much lower compared to those of pure components.
27
MICELLES --Driving Force--
- Hydrophobic groups can perturb solvent
structure and - increase free energy of system. Surfactant
will ? concentrate at - S/G interface to remove hydrophobic groups
from solution and - lower DGo.
AIR
WATER
28
MICELLES --Driving Force--
- DGo can also be decreased by aggregation into
micelles - such that hydrophobic groups are directed into
interior of - structure and hydrophilic groups face solvent.
- Decrease in DGo for removal of hydrophobic
groups from - solvent contact by micellization may be opposed
by - (i) loss in entropy of surfactant
- (ii) electrostatic repulsion for charged
headgroups - Micellization is ? a balance between various
forces which - can be influenced by certain phenomena
(Mukerjee and - Mysels, 1971).
29
MICELLES --Example Mayonnaise--
lecithin
Water matrix containing fat droplets. The
surfactant (emulsifier) is lecithin. It can
contain up to 12 g of fat in 15 ml
2 µm
http//wilfred.berkeley.edu/gordon/BLOG-images/ma
yo15.jpg
30
MICELLES --Headgroup and Chain Length--
- Klevens (1953) surfactants with linear alkyl
chains, CMC is related to number of carbons by - log10CMC b0 - b1mc
- Where
- mc is number of carbons in chain
- b0 and b1 are constants
(Hunter, Foundations of Colloid Science, p. 569,
1993, Fig 10.2.1)
31
MICELLES --Headgroup and Chain Length--
- Branching or addition of double bonds or polar
groups to alkyl chain - generally increases CMC.
- Addition of benzene ring equivalent to addition
of 3.5 carbons - (methylene groups).
- Replacement of hydrogens in alkyl chain with
fluorine initially - increases CMC, followed by marked decrease as
fluorine substitution goes to saturation.
(Hunter, Foundations of Colloid Science, p. 569,
1993, Fig 10.2.1)
32
MICELLES --Temperature and Pressure--
- For ionic surfactants there exists a critical
temperature above which - solubility rapidly increases (equals CMC) and
micelles form - ? Kraft point or Kraft temperature (TK),
- Below TK solubility is low and no micelles are
present.
(Klimpel, Intro to Chemicals Used in Particle
Systems, p. 30, 1997, Fig 22)
33
MICELLES --Temperature and Pressure--
TK
surfactant crystals
- Surfactants much less effective below Kraft
point, e.g. detergents. - For non-ionic surfactants, increase in
temperature may result in - clear solution turning cloudy due to phase
separation. This critical - temperature is the cloud point.
- Cloud point transition is generally less sharp
than that of Krafft - point.
34
MICELLES --Electrolyte--
- Addition of electrolyte significantly affects
CMC, particularly - for ionic surfactants.
- For non-ionic and zwitterionic surfactants
- log10CMC b2 b3Cs
- where Cs is salt concentration (M)
- b2 and b3 are constants for specific
surfactant, salt and - temperature.
- Change in CMC attributed to salting in or
salting out - effects. Energy required to create volume to
accommodate - hydrophobic solute is changed in electrolyte
solution due to - water-ion interactions
- ? change in activity
coefficient.
35
MICELLES --Electrolyte--
- If energy required is increased by electrolyte,
activity - coefficient of solute is increased and salting
out occurs - ? micellization is favored
and CMC decreases. - Conversely, for salting in, CMC increases.
- Effects of electrolyte depend on radii of
hydrated anions and - cations and is greater for smaller hydrated
ions, i.e. follow - lyotropic series.
- CMC depression follows order
- F- gt BrO3- gt Cl- gt Br- gt NO3-
gt I- gt CNS- - and
- NH4 gt K gt Na gt Li
36
MICELLES --Electrolyte--
- For ionic surfactants
- log10CMC b4 b5log10Cs
- where b4 and b5 are constants for a specific
ionic head group at a - particular temperature.
- Depression of CMC with increasing salt due to
double layer - compression around charged head group and
charge screening - effect between head groups in micelle.
- Different salts vary in their effectiveness,
e.g. for sodium laurate, - CMC depression follows
- PO42- gt B4O72- gt OH- gt CO32- gt SO42- gt Cl-
37
MICELLES --Electrolyte--
The effect of added salt on the CMC of SDS and
dodecylamine hydrochloride (DHC). (From Stigter
1975a,with permission)
(Hunter, Foundations of Colloid Science, p. 572,
1993, Fig 10.2.2)
38
MICELLES --Organic Molecules--
- Small amounts of organic molecules can affect the
CMC, e.g. in - aqueous solution of SDS, dodecanol (hydrolysis
product of SDS) - causes minimum in surface tension measurement.
- Solubilization of impurity in micelles causes
rise in surface tension. - Very important for detergency, stabilization
and dispersion.
go
Surface Tension
CMC
Surfactant Concentration
39
MICELLES --Organic Molecules--
- Solubilization characterized by large increase in
solubility of lipophilic (hydrophobic) organic
species above surfactant CMC. - Lipophilic organics can aid or oppose micelle
formation. Two - classes based on mode of action.
- Group A (or Type I)
- Adsorb within micelle and reduce CMC. Typically
polar - molecules, e.g. alcohols and amides.
- Effective at low concentrations.
- Short chain members adsorb near micelle-water
interface. - Longer chain members adsorb in core
- ? can influence micelle shape.
40
MICELLES --Organic Molecules--
- Free energy of micellization lowered by
screening repulsion - between charged head groups (ionic surfactants)
and/or reducing - steric hindrance (non-ionic surfactants).
- CMC depression greatest for linear species
- ? maximum when chain length approaches that of
surfactant. - Group B (or Type II)
- Modify bulk water structure around surfactant
or micelle, - usually at higher concentrations than Group A
molecules. - Structure breakers disrupt water structure
about hydrophobic - tails and increase entropy. Entropy increase
upon micelle - formation ? reduced
- ? CMC is increased.
41
MICELLES --Organic Molecules--
- Examples of structure breakers are urea,
formamide and guanidinium salts. Most effective
on non-ionic surfactants of PEO type. - Structure makers promote structuring of water,
e.g. xylose and - fructose. Conversely, CMC is reduced due to
enhanced entropy - increase upon micellization.
- At high bulk concentrations, species such as
dioxane, esters, - short-chain alcohols and ethylene glycol can
increase solubility of - monomeric surfactant, thus opposing
micellization and raising - CMC.
42
MICELLES --Aggregation Number--
- Formation of micelles from association of n
surfactant monomers can be described by - nS Mn
- where n is number of surfactant monomers needed
to form a micelle ? aggregation number - k1 and k-1 are rate constants for forward and
reverse reactions - Equilibrium constant, K, can be expressed as
k1
k-1
43
MICELLES --Aggregation Number--
If Cs and Cm are concentrations of surfactant
monomer and micelle, respectively
Variation of dCm/dCT with total surfactant
concentration for different values of aggregation
number, n. C0 is the critical micellization
concentration and Cm the concentration of
micelles.
(Hunter, Foundations of Colloid Science, p. 572,
1993, Fig 10.3.1)
44
MICELLES --Aggregation Number--
t1
t2
Micelle size distribution. Mn is the number of
aggregates of size n. The aggregates on the left
side of the minimum (L) are called submicellar,
those on the right-hand side (proper) micelles
with mean size of n, and the width of their size
distribution is given as s.
(Hunter, Foundations of Colloid Science, p. 572,
1993, Fig 10.7.1)
45
MICELLES --Aggregation Number Trends--
- For same polar group, n increases with increasing
chain - length.
- (2) For constant alkyl chain length, n decreases
with increasing number of ethylene oxide groups
in surfactant molecule. - (3) Oils or long chain alcohols increase n.
- (4) For ethoxylated non-ionic surfactants, n
drastically increases with temperature. - (5) For anionic surfactants, n increases when
NaCl is replaced with MgCl2 or CaCl2. - (6) In aqueous solution, n ranges from 50-5,000,
while in organic solvents, n usually lt 10.
(Hunter, Foundations of Colloid Science, p. 572,
1993, Fig 10.7.1)