SOD1ALS link: - PowerPoint PPT Presentation
1 / 41
Title:
SOD1ALS link:
Description:
Model studies in cell culture and ALS Tg mice and rats. WT and mutant SOD1 ... Hydro(pero)xy derivatives of aliphatic amino acids. Chloramines, deamination ... – PowerPoint PPT presentation
Number of Views:67
Avg rating:3.0/5.0
Title: SOD1ALS link:
1
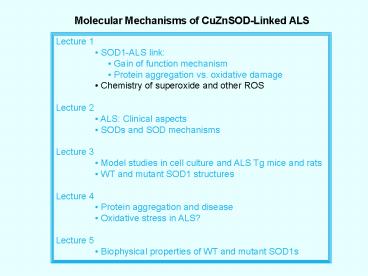
Molecular Mechanisms of CuZnSOD-Linked ALS
Lecture 1 SOD1-ALS link Gain of
function mechanism Protein aggregation
vs. oxidative damage Chemistry of superoxide
and other ROS Lecture 2 ALS Clinical
aspects SODs and SOD mechanisms Lecture 3
Model studies in cell culture and ALS Tg mice
and rats WT and mutant SOD1
structures Lecture 4 Protein aggregation and
disease Oxidative stress in ALS? Lecture 5
Biophysical properties of WT and mutant SOD1s
2
Oxygen reactions in oxidative degradation and
oxidative stress
- Air contains 21 dioxygen, O2
- Animals, plants, and aerobic bacteria require
dioxygen for efficient production of energy. - The earliest forms of life were anaerobic.
- Dioxygen is toxic to all forms of life, whether
anaerobic, aerotolerant, or aerobic. - Life coexists with dioxygen by use of of
antioxidant defense systems or by repairing or
replacing the components damaged by oxidative
stress.
3
Oxygen reactions in respiration
O2-
O2-
4
(No Transcript)
5
(No Transcript)
6
- Kinetics of dioxygen reactions
- Direct reactions of dioxygen tend to be slow
because ground state dioxygen is a triplet and
most reactants are singlets. - Triplet-to-singlet spin conversions are forbidden
by quantum mechanics and hence are slow. - A collision between two molecules occurs much
more rapidly than a spin flip and so cannot be
concerted. - Instead, the number of unpaired electrons remains
the same before and after each elementary step of
a chemical reaction, and spin flips must be
thought of as kinetically separate steps. - For these reasons, we know that it is impossible
for a spin forbidden reaction to go in one
concerted step.
- triplet singlet
7
A direct reaction of O2 in which each step is
spin allowed
3O2 (??) 1X (??) ? 2O2- (?) 2X
(?) 2O2- (?) 2X (?) ? 2O2- (?) 2X
(?) 2O2- (?) 2X (?) ? 1XO2 (??)
8
Reaction of dioxygen with reduced flavins
This type of reaction is very unusual because
most substrates are not good enough reducing
agents to make superoxide.
9
Free radical autoxidation
2 ROO. ? ROOOOR ? O2 ROOR (plus other
oxidized products such as ROOH, ROH, RC(O)R,
RC(O)H)
Much more common. Very small traces of redox
metal ions and peroxide can initiate.
10
(No Transcript)
11
ROS Hydroxyl radical and high valent metal oxo
species Highly reactive, indiscriminant oxidants
Redox metal ions usually involved in
generation Superoxide Reactive but highly
selective Most vulnerable are labile Fe-S
clusters Hydrogen peroxide Relatively
unreactive except as precursor to hydroxyl
radicals (Peroxynitrite) Controversial---not
dealt with here Dioxygen itself is not the
primary agent of oxidative stress. It is the
precursor of all of the ROS and reacts extremely
rapidly with organic free radicals, when they are
present.
12
- Hydrogen peroxide itself is a strong oxidant
thermodynamically, but its reactions tend to be
quite slow in the absence of a catalyst. - Very small traces of redox-active metal ions can
dramatically catalyze oxidation reactions of H2O2
- Fe2 H2O2 H ? Fe3 H2O HO.
- Fe3 1/2 H2O2 ? Fe2 1/2 O2 H
- __________________________________________
- 3/2 H2O2 ? H2O 1/2 O2 HO.
Hydrogen peroxide should not itself be considered
a dangerous ROS unless small traces of redox
metals, especially Fe2/3, are present.
13
- Hydroxyl radical, HO .
- One of the most reactive of the ROS known.
- It is commonly generated from reaction of H2O2
with reduced Mn (Fenton reaction). - H
- Fe2 H2O2 ? (FeIVO)2 H2O ? Fe3 H2O
HO. - Cu H2O2 H ? (CuIII-OH)2 H2O ? Cu2
H2O HO. - High valent metal-oxo or hydroxo intermediates,
e.g., (FeIVO)2 and (CuIII-OH)2, are also
implicated as ROS. - Hydroxyl radicals and high-valent metal oxo and
hydroxo species can act as initiators of free
radical autoxidation of lipids and can damage
proteins, nucleic acids, carbohydrates, and other
organic molecules when they are generated in
close proximity to such molecules. - HO. H-X ? H2O X.
14
- SUPEROXIDE, O2-
- The pK of HO2 is 4.8 in aqueous solution.
- Thus the predominant species present in solution
at physiological pH is the unprotonated
superoxide anion itself. - HO2 ? O2- H K 1.6 x 10-5 M
- Superoxide itself is a much more sluggish oxidant
than hydroxyl radical and hence it is much more
selective in the targets that it oxidizes. - The best characterized targets are ironsulfur
cluster-containing proteins containing single
labile iron atoms in their clusters.
15
- Superoxide disproportionates spontaneously to
yield hydrogen peroxide and dioxygen via a pH
dependent mechanism involving reactions 1 and 2. - Reaction 3 does not occur in the pH range of
0.2-13. - HO2 HO2 ? H2O2 O2 k 8.3 x 105 M-1s-1
(1) - H
- HO2 O2- ? H2O2 O2 k 9.7 x 107
M-1s-1 (2) - O2- O2- ? no reaction (3)
16
Rate Constants for Superoxide Dismutation
CuZnSOD
NiSOD
MnSOD
no catalyst
Rate constants for the SODs are per concentration
Cu, Mn, and Ni, respectively. (Figure courtesy of
Dr. Diane E. Cabelli.)
17
- Those rare cases in which O2- is observed to
oxidize substrates at high rates occur only when
proton transfer is simultaneous with electron
transfer, resulting in formation of HO2- rather
than O22-. - X ..... O2- ..... H-Y ? X HO2- Y-
- An example of a fast oxidation by superoxide in
which such proton-coupled electron transfer to
superoxide is likely to be occurring is the rapid
oxidation of hydroquinones by superoxide.
18
- Alternatively, a metal ion may be oxidized by
superoxide in an oxidative addition reaction to
give a metal peroxo complex, where the peroxide
is stabilized by coordination to the metal ion
rather than by protonation, followed by peroxide
dissociation, resulting in overall oxidation of
the metal ion. - In this case, the electron transfer to form a
metal-bound peroxide can precede the protonation
step because the metal ion stabilizes the O22-
ligand as it is formed. - H
- Mn O2- ? M(n1)(OO2-) ? M(n1) H2O2
19
(No Transcript)
20
ROS Hydroxyl radical and high valent metal oxo
species Highly reactive, indiscriminant oxidants
Redox metal ions usually involved in
generation Superoxide Reactive but highly
selective Most vulnerable are labile Fe-S
clusters Hydrogen peroxide Relatively
unreactive except as precursor to hydroxyl
radicals (Peroxynitrite) Controversial---not
dealt with here Dioxygen itself is not the
primary agent of oxidative stress. It is the
precursor of all of the ROS and reacts extremely
rapidly with organic free radicals, when they are
present.
21
What is protein oxidation?
- Covalent modification of a protein induced by ROS
or by-products of oxidative stress.
22
Reactivity of Proteins with ROS
- Low or no direct reactivity with superoxide or
hydrogen peroxide (in the absence of trace metals
or other catalysts) - High reactivity with OH and free radical
oxidants with similarly high reactivities (can
come from H2O2 or from lipid and other organic
peroxides). - Reactive with products of lipid peroxidation
(e.g., HNE, hydroxynonenal, and MDA,
malondialdyhyde) - Reactive with 1O2
- RNS?
- Metalloproteins can have other metal-mediated
pathways that give major oxidative damage to that
protein
23
General types of protein oxidative modification
- Sulfur oxidation (Cys disulfides, S-thiolation
Met sulfoxide) - Protein carbonyls (side chain aldehydes, ketones)
- Tyrosine crosslinks, chlorination, nitrosation,
hydroxylation - Tryptophanyl modifications
- Hydro(pero)xy derivatives of aliphatic amino
acids - Chloramines, deamination
- Amino acid interconversions (e.g., His to Asn
Pro to OH-Pro) - Lipid peroxidation adducts (MDA, HNE, acrolein)
- Amino acid oxidation adducts (e.g.,
p-hydroxyphenylacetaldehyde) - Glycoxidation adducts (e.g., carboxymethyllysine)
- Cross-links, aggregation, peptide bond cleavage
24
Amino acids most susceptible to oxidation and
their main reaction products
25
Sulfur Oxidations
- In general, Cys and Met are the amino acids that
are most susceptible to oxidation - Distinguished from other oxidative protein
modifications in that cells have mechanisms to
reverse the oxidation - e.g., methonine sulfoxide reductase
- e.g., glutathione or thioredoxin redox systems
- Hence may serve a regulatory function
- Reversible oxidation/reduction of methionine may
protect proteins from more damaging forms of
oxidative modification (e.g., carbonyl formation)
Stadtman, E. R., Moskovitz, J., Berlett, B. S.,
and Levine, R. L. (2002) Mol. Cell. Biochem.
234-235, 3-9
26
Peptide bond cleavage due to reaction with
hydroxyl radical
Peptide Bond Cleavage. OH, generated by either
radiolysis of water or the metal-catalyzed
cleavage of H2O2 can abstract hydrogen atoms from
the -CH(R)- group of the polypeptide backbone
(reactions a, b). The alkyl radical thus formed
may react with oxygen to form the alkylperoxy
radical (reaction c) or with another alkyl
radical to form inter- or intraprotein
cross-linkages (reaction p).
27
- The protein peroxy radical can be converted to
the alkyl peroxide by either - reaction with free peroxy radical (reaction d),
- reaction with Fe2 (reaction e), or
- abstraction of a hydrogen from another source
(not shown). - Irrespective of how it is formed, the protein
alkyl peroxide can be converted to the alkoxy
protein derivative by either - dismutation (reaction o),
- reaction with free peroxy radical (reaction f),
or - reaction with Fe2 (reaction g).
28
Finally, the alkoxy radical may undergo
conversion to the hydroxy derivative (reactions
i, j), which will undergo peptide bond scission
by the so-called ?-amidation pathway (reactions
k, l). Alternatively, the alkoxy radical may
undergo peptide bond cleavage by the so-called
diamide pathway (reaction m).
29
Peptide bond cleavage can occur also as a
consequence of OH attack on the side chains of
glutamyl residues leading to the overall reaction
2 or of prolyl residues according to the overall
reaction 3.
30
Figure 3 Introduction of carbonyl groups into
proteins and generation of protein-protein
cross-linkages by reaction with ,??-unsaturated
aldehydes.
31
Figure 4 Generation of protein carbonyl
derivatives and protein-protein cross-linkages by
glycation and glycoxidation reactions.
Abbreviations P-Lys-NH2, ?-NH2 groups of lysine
residues of proteins Arg-P2, arginine residue of
another protein molecule.
32
Figure 5 Metal-catalyzed oxidation of proteins by
mixed-function oxidation systems. Abbreviations
MFO, mixed-function oxidation systems RH2,
electron donors (e.g., NADH, NADPH) RCO,
protein carbonyl derivatives "OXPROT", oxidized
protein PROT, protein.
33
Reaction scheme showing how metal-catalyzed
protein oxidation can be a site-specific process
Fe (III)
Fe (II)
Peroxide or other ROS
Oxidatively damaged
Reactive intermediate
34
(No Transcript)
35
A little more about protein carbonyls
- Carbonyl groups are stable (aids detection and
storage) - Present at low levels in most protein
preparations - (1 nmol/mg protein 0.05 mol/mol 1/3000
amino acids) - See 2- to 8- fold elevations of protein carbonyls
under conditions of oxidative stress in vivo - Induced in vitro by almost all types of oxidants
(site-specific metal catalyzed oxidation,
?-irradiation, HOCl, ozone, 1O2, lipid peroxide
adducts) - Sensitive assays are available ( 1 pmol)
36
Detection of protein carbonyls
- Measure total protein carbonyls levels after
reaction with DNPH followed by spectroscopy
(A370), ELISA, or immunohistochemistry - Measure carbonyl levels in individual proteins
within a mixture of proteins (tissue samples,
cell extracts) by reaction with DNPH followed by
Western blot immunoassay
37
Measurement of total carbonyls1.
Spectrophotometric DNPH assay
2. Immunoassays for protein carbonyls e.g.,
Western blot, ELISA, immunohistochemistry
38
Proteins that contain iron-sulfur clusters play
an important role in biological systems
Rieske iron-sulfur proteins 2Fe-2S
Aconitase family 4Fe- 4S cluster
3Fe-4S cluster
39
Aconitase
Catalyzes isomerization of citrate to isocitrate
From Garrett Grisham
40
Iron-sulfur center in aconitase
4
3
1
2
Basic residue (keeps the citrate in active site)
From Lehninger Principles of Biochemistry
41
(No Transcript)