Ringopening Polymerization - PowerPoint PPT Presentation
1 / 18
Title:
Ringopening Polymerization
Description:
Ring-opening Polymerization. We will discuss the general process shown above ... Substitution decrease DH because the number of Gauche interactions ... – PowerPoint PPT presentation
Number of Views:519
Avg rating:3.0/5.0
Title: Ringopening Polymerization
1
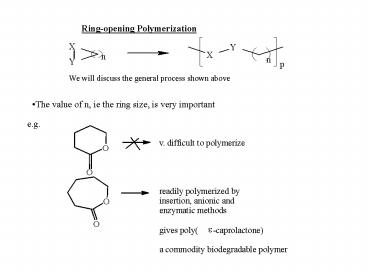
Ring-opening Polymerization
We will discuss the general process shown above
- The value of n, ie the ring size, is very
important
e.g.
2
Thermodynamic considerations
Remember ?G must be -ve for polymerization to
occur but also there must be a chemical
mechanism available. If no mechanism is
avaliable then the process will take an infinite
amount of time.
DG DH -TDS
For a ring-opening polymerization DS is -ve so
DH must be -ve in order for DG lt 0
Ring size The main influence on ?H generally
equates to ring strain. General rule-of-thumb
from organic chemistry states that 5 and
6-membered rings have small amounts of ring
strain and simple quantum mechanics calculations
support this.
3
AM1 Models give
DHf -54 kcal mol-1 -97 kcal
mol-1
4
But for the 6-membered ring
DHf -98 kcal mol-1 -96 kcal mol-1
5
So polymerizability is highly dependant on ring
size e.g.
Highly reactive To both base and acids Nuc attack
Very stable
Only polymerized by Cationic means
6
Substitution
In general substitution diminishes the ease of
polymerizability i.e. DG becomes less
negative. Substitution decrease DH because the
number of Gauche interactions increases in going
from the non-substituted polymer to the
substituted polymer. So the there are more
unfavourable interactions in the substituted
polymer than in the non-substitutedpolymer and
DH increases.
7
AM1 Models of caprolactam
Unfavorable interactions
8
AM1 Models of N-methyl caprolactam
unfavourable interactions
An AM1 model of a confirmatiom of a short chain
segment of the polymer
9
Ring substitution DHring-opening is smaller than
in the non-substituted case. So substitution
decreases polymerizability
DHf -60 kcal mol-1 -98 kcal mol-1
10
Ring-opening Polymerization
Polylactones
remember the effect of substitution
Important lactones include Caprolactone,
polylactic acid and polyglycolic acid
11
- Polymerization mechanisms
- Insertion catalysed by metals
- Cationic
- enzymatic
- Many of these polymerizations are living
- They are chain growth processes, Ie Initiation
and propagation steps - Chain growth route to Polyesters
- Narrow PDs possible
- Biodegradable
12
- Insertion
- Typical catalysts are transition metal
- organo metallics based on Sn, An, Ti
- Most widely used are
- Sn (OCO(CH2)6CH34
13
(No Transcript)
14
For propiolactone with ethyl zinc monoxide
Coordination mechanism
15
Cationic polymerization
Initial thoughts
Initiation
Propagation
Mostly ion pairs are the propagating chain
ends-Acyl oxygen scission
16
Exocyclic attack and alkyl oxygen cleavage
Initiation Propagation
Can see both mechanisms-Problems with cyclization
and transfer Initiators-AcClSbCl3,AlCl3F3CC(O)O
C(O)CF3
17
Anionic polymerization
Acyl-oxygen scission
Initiation
Propagation
Initiators
18
Polymerization of e-caprolactam
The mechanism is an activated monomer mechanism
NOT propagation by nucleophilic attack of an
open propagating chain end.
The mechanism is driven to the route shown
because the pKa of the cyclic monomer is lower
than the open chain amide.