Conversions between scales of different reference electrodes - PowerPoint PPT Presentation
1 / 17
Title:
Conversions between scales of different reference electrodes
Description:
Title: No Slide Title Author: deborah Last modified by: Information Technology Created Date: 11/3/2000 8:36:55 PM Document presentation format: On-screen Show – PowerPoint PPT presentation
Number of Views:506
Avg rating:3.0/5.0
Title: Conversions between scales of different reference electrodes
1
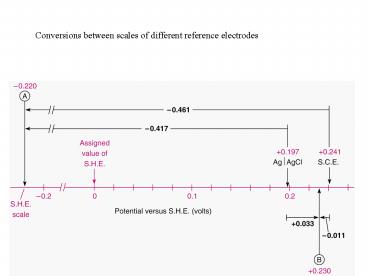
Conversions between scales of different reference
electrodes
2
Line diagram of the glass electrode
3
Combination glass electrode with silver-silver
chloride reference electrode
4
Additional models of combination pH electrodes.
5
Ion-exchange process across the glass membrane of
the glass electrode
6
- The voltage response of the glass electrode is
linear with the pH (or log aH) as shown in
equation 14-6. - E constant ?(0.05916)?pH
- The value of ? (the slope of E vs pH) is
generally 1.0. The constant is the asymmetry
potential and arises from the differences in the
two sides of the glass membrane. The asymmetry
potential is corrected by calibration of the
glass electrode against a pH buffer solution of
known pH.
7
In order to use the glass electrode to make
meaningful measurements you need to note
- standardize with standard pH buffers
- junction potentials minimize effects by matching
the ionic composition of the unknown to standards
if possible. - Drift of junction potentials recalibrate as
needed - Na error
- Acid error
- Equilibration (response time)
- Hydration of the glass membrane
- Temperature
8
Acid and sodium errors
9
Solid-state pH probe
10
Ion-selective electrodes The Fluoride electrode
11
Calibration curve for the fluoride electrode
12
Diagram of the Calcium ion-selective electrode
13
Compound electrodes are conventional electrode
systems surrounded by a membrane that reacts in a
specific way with an analyte. Pictured here is
the CO2 gas sensing electrode.
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)






























![Lecture note : Gas chromatography [2] ????????? ??? PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/6741367.th0.jpg?_=20150612015)