NET IONIC EQUATIONS - PowerPoint PPT Presentation
1 / 7
Title:
NET IONIC EQUATIONS
Description:
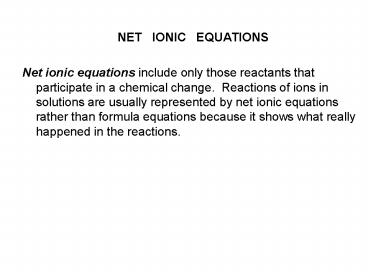
NET IONIC EQUATIONS Net ionic equations include only those reactants that participate in a chemical change. Reactions of ions in solutions are usually represented by ... – PowerPoint PPT presentation
Number of Views:56
Avg rating:3.0/5.0
Title: NET IONIC EQUATIONS
1
- NET IONIC EQUATIONS
- Net ionic equations include only those reactants
that participate in a chemical change. Reactions
of ions in solutions are usually represented by
net ionic equations rather than formula equations
because it shows what really happened in the
reactions.
2
- Start with a regular chemical equations showing
ppt. - Write ionic equation showing all dissolved
dissociated ions in solutions. - Notice Which ions appear on both sides of the
equation that have NOT under-gone chemical
change. These are called SPECTATOR IONS. - To convert an ionic equation to a NET ionic
equations the spectator ions are canceled on both
sides of the equation. - 4. Final Net Ionic Equation only shows active
ions and the ppt.
3
- Write chemical, complete ionic, and net ionic
equations for the following. - Solutions of silver nitrate and sodium chloride
are mixed.
4
- 2. Solutions of lithium phosphate and copper II
sulfate are mixed.
5
- Solutions of aluminum chloride and ammonium
hydroxide are mixed.
6
- Solutions of iron II sulfate and barium carbonate
are mixed.
7
- 5. Solutions of zinc nitrate and ammonium
sulfide are mixed.