- PowerPoint PPT Presentation
1 / 1
Title:
1
Acid Stress Suppresses The Thioglycollate-Stimulat
ed Inflammatory Response of Adult Rana
pipiens Matthew J. Colombo, Jaime L. Andrews,
Emily J. Piddington, Muthuramanan Rameswaran,
Elizabeth Fiorini, Itzick Vatnick, Marc A.
Brodkin Department of Biology, Widener
University, Chester, PA 19013
ABSTRACT Acidic environments function as a
stressor for amphibians. Previous work in our lab
showed that adult ranid frogs exhibit different
levels of tolerance to mild acid conditions (pH
5.5). Adult Rana pipiens are more susceptible
than other ranids and experienced 72 mortality
after ten-day exposure to pH 5.5. We also
demonstrated that acid exposure increases the
permeability of the gut to the endogenous
bacterial flora and results in a systemic
infection. Inflammation is a natural protective
defense against microbial infection,
characterized by an influx of leukocytes to the
site of infection. Thioglycollate medium is used
experimentally to stimulate an inflammatory
response in several vertebrates. Intra-peritoneal
injection of thioglycollate causes a peritoneal
exudate containing leukocytes. Our study shows
that acid exposure (pH 5.5) greatly suppresses
the inflammatory response in thioglycollate-induce
d R. pipiens. The number of leukocytes as well
as their phagocytic efficiency was significantly
reduced in acid-exposed thioglycollate-stimulated
adult R. pipiens as compared to their controls.
Frogs induced by thioglycollate injection and
exposed to pH 5.5 had a 50 increase in cells
that did not exhibit phagocytosis and a four-fold
reduction in the number of highly efficient
phagocytic cells.
INTRODUCTION In the late 1970s, amphibians began
to disappear in various locations around the
globe. The causes of this global decline are not
yet known, however pathogens seems to be involved
in all instances of reported extinctions. In the
Northeastern United States, R. pipiens enter
hibernation in late October to early November and
emerge from hibernation in early spring. Soon
after their emergence, they mate and lay their
eggs in late spring to early summer. These frogs
emerge from hibernation with compromised immune
systems (Cooper et al., 1992) to mate in
surrounding streams and ponds. It is during
this post-hibernation period when streams, ponds,
and lakes in the northeastern United States
suffer from episodic acidification due to
snowmelt and rain events (Driscoll et al., 2001).
Therefore, acidification of aquatic ecosystems
may contribute to the declining populations of R.
pipiens. The effect of acid exposure on the
inflammatory response was examined.
Inflammation is a natural protective defense
against microbial infection, characterized by an
influx of leukocytes to the site of infection.
Thioglycollate has been shown to cause this
response in several other animal models (Menaszek
et al., 1999 Melnicoff et al., 1989 Cadzinska
et al., 1997). Using this experimental technique,
we developed an assay to probe the mechanism of
the effect of acid exposure on the inflammatory
response of adult Rana pipiens. To further
characterize this response, FITC-labeled 1.0
micron polystyrene beads were infused into the
thioglycollate inoculation to serve as surrogates
for bacteria. These allowed for the measurement
of the phagocytic efficiency of activated
leukocytes.
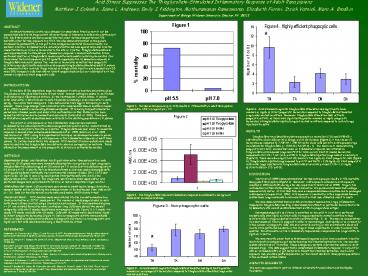
Figure 1. Ten-day acid exposure (pH 5.5)
results in 72mortality in adult Rana pipiens,
compared to 3.5 in frogs at pH 7.0.
Figure 4. Acid-stressed frogs with thioglycollate
stimulation had significantly lower numbers of
highly efficient phagocytic leukocytes compared
to thioglycollate-stimulated frogs under neutral
conditions. Moreover, thioglycollate stimulation
does activate phagocytic activity, as there were
significantly greater numbers of highly
phagocytic leukocytes in thioglycollate-injected
frogs at neutral conditions compared to control
frogs injected with saline.
RESULTS Thioglycollate-inoculated Rana pipiens
exposed to a neutral pH (7.0) had 5.09E05
1.8E05 white blood cells per ml of lavage, a
six-fold increase (figure 2) in the number of
leukocytes as compared to 7.70E04 2.9E04
white blood cells per ml in acid-exposed frogs
inoculated with thioglycollate, p lt 0.00136
(F6.89, df 3). The bead count, demonstrating
phagocytic efficiency, showed that when an
inflammatory response was initiated by
thioglycollate, acid-exposed frogs had 79.17
8.86 non-efficient (0 beads) leukocytes compared
to 52.33 4.60, (U6, plt0.05) in
thioglycollate-injected frogs held at a neutral
pH (figure 3). There was also a significant
difference in the highly efficient phagocytic
cells (figure 4). Thioglycollate-injected frogs
exposed to pH 7.0 had 9.67 2.28 highly
efficient phagocytic cells, while frogs injected
with thioglycollate held at an acidic pH had
2.33 1.13 highly efficient cells.(U33.5,
plt0.01).
METHODS Experimental Groups and Conditions.
Adult, post-hibernation Rana pipiens with a body
weight of 15-30 grams were were randomly
allocated into four groups of eight frogs each
thioglycollate-inoculated frogs at pH 5.5 and at
pH 7.0, and buffer-inoculated frogs at pH 5.5 and
at pH 7.0. A sterile citric acid/sodium citrate
buffer was used and changed daily. All frogs were
placed individually in an environmental chamber
(6 days, 25o C, 12/12 day-night cycle). On Day
5, each frog was injected intra-peritoneally with
2 ml of the appropriate solution. On Day 6, the
frogs were sacrificed by ether asphyxiation, and
peritoneal lavages were performed with 10 ml of
an isotonic amphibian ringer solution. White
Blood Cell Count. Cell counts were performed by
pipetting the lavage fluid onto a hemacytometer
and by multiplying the average number of
leukocytes per 1 mm field by 104 (n 31). Data
for total leukocyte counts were analyzed by ANOVA
and a t-test. Fluorescent Beads. FITC-labeled
beads were diluted into the inoculation medium to
a final concentration of 2.5107 beads per ml.
The number of beads phagocytosed by each white
blood cell was counted using a fluorescence
microscope. In this experimental group, n 24,
one hundred leukocytes were counted per frog.
Cells were placed into several categories cells
with 0 beads (classified as non-phagocytic cells,
figure 3), 1-3 beads, 4-6 beads, 7-9 beads, and
cells with gt10 beads. Cells with gt10 beads were
classified as highly efficient phagocytic
leukocytes (figure 4). Data for phagocytic
activity were analyzed with a Chi square analysis
and differences in phagocytic efficiency of
non-phagocytic and highly efficient cells was
discerned using a Mann-Whitney U-test.
DISCUSSION Vatnick et al. (1999) demonstrated
that ten-day acid exposure results in 72
mortality among adult Rana pipiens (Figure 1).
Moreover, cold-exposure followed by acid exposure
resulted in 100 mortality during a ten day
experiment (Vatnick et al. 1999). Frogs in the
northeastern United States emerge from
hibernation into ponds and streams that undergo
seasonal acidification. At this time they have a
compromised immune system due to prolonged cold
exposure (Cooper et al., 1992). Acid exposure in
combination with a suppressed immune system leave
frogs vulnerable to microbial infection that may
ultimately result in death. We have demonstrated
that an acidic environment weakens the frogs
inflammatory response to thioglycollate resulting
in decreased recruitment of peritoneal leukocytes
to the inflammatory site, as well as diminishes
their ability to phagocytose antigen.
Decreased phagocytic efficiency is exhibited by
the shift in function of peritoneal exudate cells
from highly efficient cells in frogs subjected to
normal conditions to less efficient cells in
frogs subjected to acidic conditions (Figs. 3,
4). This may be caused either by a decreased
efficiency of phagocytic cells (i.e. macrophages,
neutrophils) or by a reduction in the number of
phagocytic cells. We are currently working on
differential white blood cell counts in the
peritoneal exudates of the frogs in these
experiments in order to answer this question. The
attenuation of the inflammatory response may
compromise the frogs ability to fight off
infection We have recently shown that
acid-stressed frogs may suffer from a systemic
infection due to transit of endogenous gut
bacteria across the intestinal epithelium into
the vascular system (Brodkin et al., in review).
These endogenous bacteria colonized the spleens
of acid-exposed frogs. This data suggest that a
compromised inflammatory response, in conjunction
with an increased ability of gut bacteria to
transit the intestinal epithelium, due to acid
exposure may provide a partial explanation for
the recent decline in Rana pipiens populations in
the northeast United States.
Figure 2. The thioglycollate-induced inflammatory
response is abolished to background levels after
six-day acid stress.
REFERENCES Cadizinska, M., Jozefowski, S., Gigaj,
J., and Plytycz, B. 1977. Morphine modulation of
thioglycollate elicited peritoneal inflammation
in Goldfish. Cariasius auratus. Archivum
Immunologiac Experimentalis 45321-327. Cooper
EL, Wright RK, Klempau AE, Smith CT. 1992.
Hibernation alters a frogs immune system.
Cryobiology, 29616-631. Driscoll CT, Lawrence
GB, Bulgar AG, Butler TJ, Cronan CS, Eagar C,
Lambert KF, Likens GE, Stoddart JL, Weathers KC.
2001. Acidic deposition in the northeastern
United States sources and inputs, ecosystem
effects, and management strategies. Bioscience
51180-198. Melnicoff, M., Horan, P., and
Morahan, P. 1989. Kinetics of changes in
peritoneal cell populations following acute
inflammation. Cellular Immunology 118
178-91. Menaszek, E.Miskiewicz, K. and Plytycz,
B. 1999. Comparative studies on experimental
inflammations in anuran amphibians.
Central-European Journal of Immunology
24211-217. Vatnick I, Brodkin MA, Simon MP,
Grant BW, Conte CR, Gleave M, Myers R, Sadoff MM.
1999. The effects of exposure to mild acidic
conditions on adult frogs (Rana pipiens and Rana
clamitans) Mortality rates and pH preferences.
J Herp 33370-374.
ACKNOWLEDGMENTS This work was supported in
part by Widener University Provosts Grant and
the Eppley Foundation.
Figure 3. Acid-stressed frogs with
thioglycollate stimulation had significantly
greater numbers of non-phagocytic leukocytes
compared to thioglycollate-stimulated frogs under
neutral conditions.