Elimination Reactions PowerPoint PPT Presentation
Title: Elimination Reactions
1
Part 4 Elimination Reactions
2
Learning Objectives Part 4 Elimination
Reactions
CHM1C3 Introduction to Chemical Reactivity of
Organic Compounds
- After completing PART 4 of this course you should
have an understanding of, and be able to
demonstrate, the following terms, ideas and
methods. - (i) Understand E2 and E1 reaction mechanisms
- (ii) Understand how experimental evidence from
rate equations and stereochemical outcomes in the
product lead to the proposal of reaction
mechanisms - (iii) Understand the experimental factors which
favour E2 or E1 reaction mechanisms - (vi) Understand the term antiperiplanar in the
context of E2 reaction mechanisms - (v) Understand that in assessing the reaction
outcome in an elimination reaction, the
stereoelectronic of the alkylhalide needs to be
considered carefully, ie. the constitution and
conformation
3
Elimination Reactions
Clearly, two different reaction mechanisms must
be in operation. It is the job of the chemists
to fit the experimental data to any proposed
mechanism
4
(No Transcript)
5
The E2 Reaction Mechanism
Compare to SN2
6
(No Transcript)
7
The E1 Reaction Mechanism
Compare to SN1
8
Reactive Intermediate
9
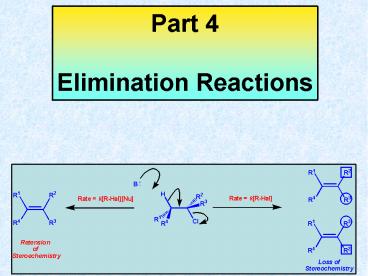
Stereochemistry Compared
E1
E2
H, C, C and Cl are antiperiplanar
10
(No Transcript)
11
Alkene Stability
Stability Increases
12
Constitutionally Different Eliminations
Base
Constitutional Isomers
Statistically favoured!
13
High Energy Transition State
Conformational Equilibria
Diastereoisomers
Conformers
Low Energy Transition State
14
High Energy Transition State
Low Energy Transition State
15
Cyclohexane Rings E2
Two C-H bonds are antiperiplanar to the C-Cl bond
16
Cyclohexane Rings E1
No C-H bonds are antiperiplanar to the C-Cl bond
17
Summary Sheet Part 4 Elimination Reactions
CHM1C3 Introduction to Chemical Reactivity of
Organic Compounds
The difference in electronegativity between the
carbon and chlorine atoms in the C-Cl sigma (?)
bond result in a polarised bond, such that there
is a partial positive charge (?) on the ?-carbon
atom and a slight negative charge (?-) on the
halogen atom, which in turn is transmitted to the
?-carbon atom and the protons associated with it.
Thus, the hydrogen atoms on the ?-carbon atom
are slightly acidic. Thus, if we react
haloalkanes with bases (chemical species which
react with acids), the base will abstract the
proton atom, leading to carbon-carbon double bond
being formed with cleavage of the C-Cl bond. The
mechanism of this ?-elimination (or 1,2
elimination) can take two limiting forms
described as Bimolecular Elimination (E2) and
Unimolecular Elimination (E1). The E2
mechanism fits with a rate equation which is
dependent on both the base and haloalkane, and
that the product retains the stereochemical
information about the C?-C? bond. This retension
of stereochemical integrity requires an
antiperiplanar relationship of the eliminated
atoms. In contrast, The E1 mechanism fits with a
rate equation which is dependent on only the
haloalkane, and that the product undergoes a loss
of the stereochemical information about the C?-C?
bond. Thus, with appropriately substituted
haloalkane a pair of diastereomeric alkenes are
formed, as result of rotation around the C?-C?
bond upon formation of the carbocationic
intermediate.
18
(No Transcript)
19
Exercise 1 Substitution/Elimination Reactions
Rationalise the experimental results that when 1
is reacted with NaOEt in EtOH, two alkenes are
formed, whereas 2 under the same conditions
affords an inverted substitution product.
20
Answer 1 Substitution/Elimination Reactions
Rationalise the experimental results that when 1
is reacted with NaOEt in EtOH, two alkenes are
formed, whereas 2 under the same conditions
affords an inverted substitution product.
As 2 undergoes an inversion of stereochemistry
one must assume SN2 mechanism. As 1 is subject to
the same reaction conditions as 2 one must assume
that elimination of HCl does not involve the
formation of a carbocation, and thus E2 mechanism
must operate.
SN2
E2
E2
21
Exercise 2 Elimination Reactions
Rationalise the following
22
Answer2 Elimination Reactions
Rationalise the following
The energy to attain this transition state TS2
geometry is much higher that TS1, because the
largest substituent (t-Bu) and the Cl are both in
the axial positions, which leads to large steric
clashes. Thus, more energy, i.e. higher
reaction temperatures, are required to attain TS2
relative to TS1.