Matter and Measurement - PowerPoint PPT Presentation
Title:
Matter and Measurement
Description:
Chemical Equilibrium N2(g) + 3 H2(g) -- 2 NH3(g) Many reactions do not go to completion - under the given conditions it is possible that not all of the reactants are ... – PowerPoint PPT presentation
Number of Views:50
Avg rating:3.0/5.0
Title: Matter and Measurement
1
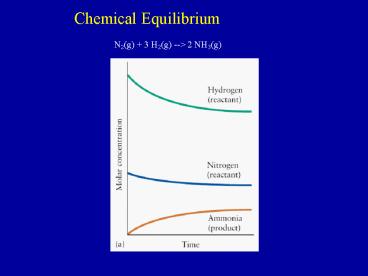
Chemical Equilibrium
N2(g) 3 H2(g) --gt 2 NH3(g)
2
- Many reactions do not go to completion - under
the given conditions it is possible that not all
of the reactants are consumed. - Instead the extent of the reaction is determined
by the equilibrium point. - A B --gt C D
- As the concentrations of C and D increase, C and
D could react to form A and B - the REVERSE
reaction.
N2(g) 3H2(g) 2NH3(g)
3
- The forward and reverse directions oppose one
another. - At some point in time, the rate of the forward
reaction will equal the rate of the reverse
reaction - this point corresponds to EQUILIBRIUM. - Hence, when equilibrium has been reached, the
concentration of A, B, C and D stay constant, as
long as the conditions are held the same. - At the equilibrium point A and B combine to form
C and D C and D combine to form A and B but
both occur at the same rate. - There is no NET change in the concentrations of
A, B, C D.
4
Equilibrium
- When opposing forces acting on a system are equal
in magnitude, the system is said to be in a state
of equilibrium. - A dynamic equilibrium is one at which changes to
the system do occur at the microscopic level, but
at the macroscopic level these changes are not
observed. - In general Processes not at equilibrium will act
or react to reach equilibrium.
5
(No Transcript)
6
- Characteristics of equilibrium
- 1) The attainment of equilibrium is spontaneous
i.e. it is a natural tendency - 2) At equilibrium there is no macroscopic
evidence of any changes in the system - 3) A dynamic balance is established between
opposing forces - 4) Equilibrium is reached from either direction
7
- The Equilibrium Expression
K (constant)
K is called the EQUILIBRIUM CONSTANT
Note K has a fixed value for a particular
reaction and varies with temperature
8
- If all reactants and products are gases, the
relationship between the partial pressures of all
gases at equilibrium is
If all reactants and products are in solution,
the relationship between the concentrations of
all species at equilibrium is
Where X is the concentration (example molarity)
of species X at equilibrium
Homogenous reactions reactants and products in
the same phase
9
- Heterogeneous reaction reactants and products
are not in the same phase
a A(aq) b B(aq) c C(aq) d D(g)
10
K is a dimensionless quantity. 2A(g)
B(g) K PB/P2A is actually
PB/Pref
K
(PA/Pref)2
Pref is set to 1 atm K is dimensionless For
solutions, if concentration is M Aref 1M
11
HCl(aq) H(aq) Cl-(aq)
H Cl-
K
K 107 at 25oC
HCl
CH3COOH(aq) H(aq) CH3COO-(aq)
K 10-5 at 25oC
12
(No Transcript)
13
The reaction of SO2(g) and O2(g) forming SO3(g)
Equilibrium can be reached for different partial
pressures of SO2, O2, and SO3, depending on the
starting conditions, but at 25oC, the value of K
is the same.
14
- The Magnitude of the Equilibrium Constant
- The magnitude of the equilibrium constant reveals
the extent to which the reaction will proceed in
the desired direction.
Reactions that have K values gt 1 are favored in
the direction written i.e. forward
direction. Reactions that have K values lt 1 are
favored in the reverse direction Reactions for
which K is near I have substantial amounts of
both reactants and products when equilibrium is
established.
15
(No Transcript)
16
- Applying the Equilibrium Expression to Gas Phase
Reactions
What are the equilibrium partial pressures of all
three gases in a closed container containing only
PCl5 at 0.100 atm and held at 250oC? According
to the ideal gas laws, the partial pressures of
gases is proportional to the number of moles of
each gas, as long as the volume and temperature
are kept fixed. The stoichiometry of this
reaction is 1 1 1
17
If the partial pressure of PCl5 decreases by x at
equilibrium, the partial pressures of PCl3 and
Cl2 increases by x at equilibrium.
At equilibrium
18
x2 2.15 (0.100-x) x2 2.15x - 0.215 0
- This is a quadratic equation of the form
- ax2 bx c 0
- and the solution of this equation is of the form
19
Using this expression and solving for x, the
roots of the equation are x 0.0957 and -2.25
atm. At equilibrium, the partial pressures of
Cl2 and PCl3 are 0.0957 atm, and that of PCl5 is
(0.100 - 0.0957) 0.004atm
20
The three gases are introduced into a container
at partial pressures of 3.6 atm NO2, 5.1 atm N2O
and 8.0atm O2 and react to reach equilibrium at a
fixed temperature. The equilibrium partial
pressure of NO2 is measured to be 2.4 atm.
Calculate the equilibrium constant of the
reaction at this temperature, assuming that no
competing reactions occur.
21
Initial P (atm)
3.6 5.1 8.0
Change in P (atm)
- x 2x/4 3x/4
Change in P (atm)
- 4x 2x 3x
Equilibrium P (atm)
2.4 5.12x 8.03x
- At equilibrium, the partial pressure of NO2 is
2.4 atm - 3.6 - 4x 2.4 gt x 0.3 atm
- Hence PN2O at equilibrium 5.7 atm PO2 8.9
atm
22
- In applying the equilibrium expression the
following must be considered. - 1) The equilibrium constant for a reverse
reaction is the reciprocal of the equilibrium
constant for the corresponding forward reaction.
23
- 2) When the coefficients in a balanced chemical
equation are multiplied by a constant factor, the
corresponding equilibrium constant is raised to
the power equal to that factor.
K2
PH2 PO2
1/2
24
- 3) When chemical equations are added or
subtracted to obtain a net equation, the
corresponding equilibrium constants are
multiplied or divided to obtain the equilibrium
constant of the net equation.
25
- Adding the two chemical equations gives
Looking at the expressions for K1, K2 and K3 K1
K2 K3 Hence, K3 0.023 at 25oC
26
- The Ideal Gas Equation and Chemical Equilibrium
- For gaseous reactants or products, the
concentration may be in moles/liter. - The concentrations of the gases in moles/liter
must be converted to partial pressures.
Concentration of a species A in moles/lit A
This equation can be used to convert
concentration of a gas in moles/lit to partial
pressure of the gas.
27
Hence, for a general gas phase reaction
- where Pref is the reference pressure 1 atm and
ensures that K is unitless.
28
Reaction Quotient
- For the general reaction
Define the reaction quotient, Q
where P is the partial pressure of a species at
any point in time.
29
- If Q K, the reaction is at equilibrium
- If Q ? K, the reaction is not at equilibrium.
- If Q gt K , reaction proceeds from right to left
- If Q lt K, reaction proceeds from left to right
30
(No Transcript)
31
The equilibrium constant for the reaction CH4(g)
H2O(g) CO(g) 3 H2(g) Equals 0.172 at
900K. The concentrations of H2(g), CO(g), and
H2O(g) in an equilibrium mixture of gases all
equal 0.00642 mol/L. Calculate the concentration
of CH4(g) in the mixture, assuming that this is
the only reaction taking place.
0.00642 mol/L 0.00642 mol/L 3
CH4 0.00642 mol/L
0.172
(0.08206 L atm mol-1) 900 K-2
CH4 0.00839 mol/L
32
- What happens if a system at equilibrium undergoes
a change in conditions? - The tendency of a system to achieve equilibrium
is spontaneous. - Once a system is at equilibrium it will remain at
equilibrium. - However, if conditions change the system will
respond to this change in a way to achieve
equilibrium again. - Note the concentrations of species when
equilibrium is re-established need not be the
same as the ones established at the previous
equilibrium.
33
(No Transcript)
34
- LeChateliers Principle
- If a stress is applied to a system at
equilibrium, the system tends to react so that
the stress is minimized
A) Changing the concentration of a reactant or
product If reactant added Q lt K, reaction
proceeds from left to right If product added Q gt
K, reaction proceeds from right to left If a
product is removed from the equilibrium mixture,
Q also decreases, and the reaction once again
proceeds to the right to increase the
concentration of the product.
35
(No Transcript)
36
- B) Changing the Volume
- Decreasing the volume, increases the total
pressure of the reaction mixture. - The reaction will then proceed in the direction
which reduces the total pressure.
If the volume is decreased, the above reaction
will move to the right, to decrease the total
number of molecules.
37
- C) Changing Temperature
- The effect of changing the temperature of a
system at equilibrium depends on whether the
reaction proceeds by absorbing energy
(endothermic) or by releasing energy
(exothermic). - An endothermic reaction lowers the temperature of
the system and an exothermic reaction raises the
temperature of the system.
Forward reaction is exothermic reverse reaction
is endothermic.
If a reaction is exothermic, raising the
temperature causes the equilibrium to shift to
the left.
38
- Heterogeneous Reactions
In all equilibrium expressions, the
concentrations of all pure solids and liquids are
set to 1.
39
- In general, to write the equilibrium expression
for a reaction - 1) Concentration of gases are expressed as
partial pressures - 2) Concentration of dissolved species in solution
are expressed as moles/liter - 3) Concentrations of pure solids and pure liquids
are set to 1 - (for a solvent taking part in a reaction, its
concentration is also set to 1 providing the
solution is dilute)
40
(No Transcript)
41
42
Applications of Chemical Equilibria
Product yields can be increased by adjusting
conditions
N2(g) 3H2(g) 2NH3(g) heat
Gas phase reaction 4 moles of gas --gt 2 moles of
gas Exothermic reaction Reaction conditions that
favor NH3(g) production high pressure ( 250
atm) low temperature (use a catalyst)
43
(No Transcript)
44
(No Transcript)
45
Hemoglobin/O2 Equilibrium
- Hemoglobin (Hb) carries oxygen from the lungs to
the body tissue, transporting oxygen from a
region of high concentration to low
concentration. - The oxygen-hemoglobin complex, oxyhemoglobin
(HBO2) transports O2
Level of O2 in blood is increased by 70 times
because of hemoglobin
46
Because of the formation of the HbO2 complex, the
amount of O2 in blood is increased by a factor of
70.
LeChateliers principle predicts that in regions
of high O2 partial pressure, the Hb-HbO2
equilibrium is shifted to the right, which is the
case in the lungs In regions of low O2 partial
pressure, the equilibrium shifts to the left,
resulting in a breakup of the HbO2 complex,
releasing O2 to the bodys tissues.
47
Why is CO lethal?
48
When Hb is exposed to both O2 and CO, there is
competition for the Hb, and the following
reaction takes place
The Kcompetition for this reaction is
49
Since KCO gt KO2 Kcompetition is gt1 At 38oC, the
value of Kcompetition is 210 strongly favoring
the formation of the HbCO complex CO displaces O2
from the HbO2 complex, resulting in asphyxiation.
The process is reversible - from LeChateliers
principle, a large partial pressure of O2 will
shift the reaction above from right to left.
50
- Extraction and Separation
A solute dissolved in a solvent A can be
extracted using another solvent B. Condition
The solute must dissolve in both solvents A B
and solvent B must be immiscible with the solvent
A. CCl4 and H2O are immiscible. I2(s) dissolves
in both solvents.
51
- If to an aqueous solution of I2(aq) CCl4 is
added, and the flask shaken, some of the I2 in
the water layer will be extracted into the CCl4
layer
The following equilibrium is established
K partition coefficient
52
Chromatography
53
(No Transcript)
54
(No Transcript)