Synthesis Reactions PowerPoint PPT Presentation
Title: Synthesis Reactions
1
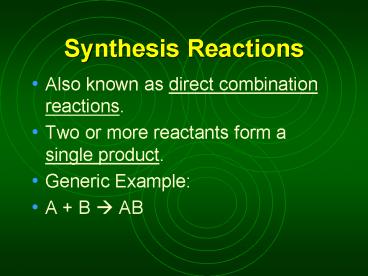
Synthesis Reactions
- Also known as direct combination reactions.
- Two or more reactants form a single product.
- Generic Example
- A B ? AB
2
Synthesis Reactions
K1 and Cl-1
Example K Cl2 ?
KCl
2
2
Remember to Balance!
3
Synthesis Reactions
Al3 and Br-1
Example Al Br2 ?
AlBr3
3
2
2
Remember to Balance!
4
Decomposition Reactions
- A single reactant is broken down into two or more
products. - Generic Example
- AB ? A B
5
Decomposition Reactions
Diatomic!!!
2
Example NaCl ?
Cl2
2
Na
Remember to Balance!
6
Decomposition Reactions
Diatomic!!!
O2
Example Al2O3 ?
3
2
Al
4
Remember to Balance!
7
Single-Replacement Rxns
- A single element replaces an element in a
compound to form a new compound and element. - Generic Example
- A BX ? AX B
Click here for a video that shows Ag replacing Cu
8
Single-Replacement Rxns
- You must use the Activity Series to determine if
a reaction will work! - Metals will replace other metals that appear
below them - (Pg. 295)
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
9
Single-Replacement Rxns
Mg2 and NO3-1
Mg(NO3)2
Ag
2
Mg AgNO3?
2
Replaced Metal
Mg is above Ag, so the reaction will work!
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
10
Single-Replacement Rxns
No Reaction
NR
Cu FeSO4?
Cu is below Fe, so the reaction will NOT work!
http//cwx.prenhall.com/bookbind/pubbooks/hillche
m3/medialib/media_portfolio/text_images/CH04/FG04_
15.JPG
11
Single-Replacement Rxns
- Non-metals will replace other non-metals that are
below them on the periodic table.
http//www.avon-chemistry.com/ch9_chart11.jpg
12
Single-Replacement Rxns
K1 and Cl-1
KCl
I2
2
Cl2 KI?
2
Diatomic!
Cl is above I, so the reaction will work!
http//www.avon-chemistry.com/ch9_chart11.jpg
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.