QUESTION: - PowerPoint PPT Presentation
QUESTION:
At regular time intervals, a fixed volume of the mixture is pipetted into large volume of ice-water to quench the reaction. (DO NOT QUENCH by Using NaOH!) – PowerPoint PPT presentation
Title: QUESTION:
1
QUESTION
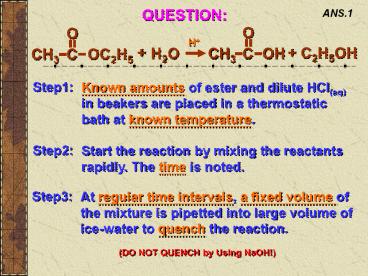
ANS.1
Step1
Known amounts of ester and dilute HCl(aq) in
beakers are placed in a thermostatic bath at
known temperature.
Step2
Start the reaction by mixing the reactants
rapidly. The time is noted.
Step3
At regular time intervals, a fixed volume of the
mixture is pipetted into large volume of
ice-water to quench the reaction.
(DO NOT QUENCH by Using NaOH!)
2
QUESTION
ANS.2
Step4
Remaining CH3COOH in the reaction mixture is
determined by titration against standard NaOH
using phenolphthalein.
Step5
Repeat steps 3 and 4 to obtain variation of
CH3COOH against t.
3
p.01
Physical Method for Studying the Variation of A
with Time
lt COLORIMETRY gt
lt COLORIMETRY gt
C. Y. Yeung (CHW, 2009)
4
p.02
Colorimetry
Monitoring the change of conc. along with time by
absorbance ? coloured substance
colour intensity
5
p.03
(for coloured reactant)
6
p.04
Examples
Br2 HCOOH ? 2Br- 2H CO2
I2 CH3COCH3 ? CH3COCH2I H I-
BrO3- 5Br- 6H ? 3Br2 3H2O
H2O2 2I- 2H ? I2 2H2O
7
p.05
Procedures
Calibration of Colorimeter Measurement
Calibration of Colorimeter
Step1
Use distilled water as sample, the absorbance is
set to zero.
Step2
A standard solution of Br2(aq) e.g. 1.0M is put
into the sample cell in the colorimeter.
Step3
Record the absorbance of 1.0M Br2(aq).
Step4
Repeat steps 2 and 3 with different Br2(aq).
8
p.06
Calibration of Colorimeter (continue)
Step5
Plot a Calibration Curve of Absorbance versus
Br2(aq).
9
p.07
Measurement
Br2 HCOOH ? 2Br- 2H CO2
Put the Br2 containing reaction mixture in the
sample cell and put into the colorimeter.
Step1
Start the stop watch to monitor the variation of
absorbance with time.
Step2
10
p.08
Monitoring the change of conc. along with time
Measurement
Calibration
A1
A1
Br2t1
t1
Time
Br2
11
p.09
Another Physical Method
Monitoring the change of conc. along with time by
12
Example
p.10
Mg 2HCl ? MgCl2 H2
(no colour change!)
13
p.11
Next .
5 Factors affecting the Rate of Rxn (p.15-21)
Rate Eqns Order of Rxn (p.25-36)
Rate Eqns Order of Rxn (p.37-43)
14
p.12
Assignment
p.24 Ex. Q.1-5 due date 11/2(Wed)
Pre-lab due date 12/2(Thur)
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.