AP BIOLOGY - PowerPoint PPT Presentation
1 / 49
Title: AP BIOLOGY
1
- AP BIOLOGY CHEMISTRY/BIOCHEMISTRY
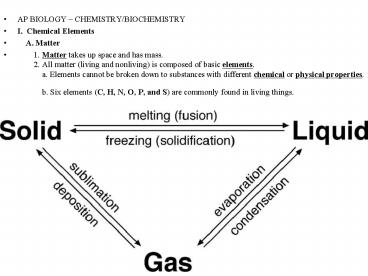
- I. Chemical Elements
- A. Matter
- 1. Matter takes up space and has mass.
2. All matter (living and nonliving) is
composed of basic elements. a.
Elements cannot be broken down to substances with
different chemical or physical properties.
b. Six elements (C, H, N, O, P, and
S) are commonly found in living things.
2
- B. Atomic Structure
- 1. Chemical and physical properties of
atoms (e.g., mass) depend on the subatomic
particles. a. Different atoms
contain specific numbers of protons, neutrons,
and electrons. b. Protons and
neutrons are in the nucleus of atoms electrons
move around the nucleus. c. Protons
are positively charged particles neutrons have
no charge both have about 1
atomic mass unit of weight. d.
Electrons are negatively charged particles.
2. Isotopes have different mass.
a. Isotopes are atoms with the same
number of protons but differ in the number of
neutrons e.g., a carbon atoms
has six protons but may have more or less than
usual six neutrons. b. Isotopes have
many uses 1) Determine diet of
ancient peoples by determining proportions of
isotopes in mummified or
fossilized human tissues. 2)
Used as tracers of biochemical pathways.
3) Determine age of fossils
using radioactive isotopes. 4)
Radiation used in medical treatment.
5) Source of radiation used in
medical diagnostic procedures including PET scan.
3
- C. Energy Levels
- 1. Protons are positively charged
electrons are negatively charged. Oppositely
charged protons and electrons are
attracted to each other. 2. An atom's
proton number determines its number of electrons
and its chemical properties. 3.
Arrangement of an atom's electrons is determined
by total number of electrons and electron shell
they occupy. a. Energy is the
ability to do work. b. Electrons
with least amount of potential energy are located
in K shell closest to nucleus electrons having
more potential energy are
located in shells farther from the nucleus.
c. Atomic Configurations
1) Bohr model helps determine
number of electrons in outer shell.
2) Inner shell contains up to
two electrons additional shells contain eight
electrons. 3) Elements are
arranged in rows in periodic table according to
number of electrons in outer shell.
d. How atoms react with one another depends upon
the number of electrons in outer shell.
1) Atoms with filled outer
shells do not react with other atoms.
2) In atom with one shell, outer
shell is filled when it contains two electrons.
3) For atoms with more than one
shell, the octet rule applies outer shell is
stable when it contains
eight electrons. 4) Atoms with
unfilled outer shells react with other atoms so
each has stable outer shell. 5)
Atoms give up, accept, or share electrons in
order to have a stable outer shell.
e. Electron Orbitals 1) Orbital
is a volume of space where rapidly moving
electrons are predicted to be found.
2) An orbital has a
characteristic energy state and a characteristic
shape. 3) At first energy level
(K shell), there is only one spherically shaped
orbital where at most two electrons
are found about the nucleus.
4) At second energy level (L
shell), there is one spherically shaped orbital
and three dumbbell shaped orbitals
the second energy level
contains at most eight electrons.
5) Higher energy levels may
contain more orbitals however, outer shells have
a maximum of four orbitals
and eight electrons.
4
- 4.Chemical Formulas and Equations
a. A chemical formula indicates the
number of atoms in each substance H2O has TWO
Hydrogen (H) Atoms and ONE
Oxygen (O) Atom. b. The formula also
indicates the number of molecules 6H2O is six
molecules of water. c. A chemical
equation is always balanced the same number of
each type of atom is on both sides. - II. Compounds and Molecules
- A. Molecules
- 1. Molecules are atoms held together by
chemical bonds. 2. Molecules form when
two or more atoms react with one another (e.g.,
O2). 3. Two or more different elements
react or bond together to form a compound (e.g.,
H2O). 4. Electrons possess energy bonds
that exist between atoms in molecules contain
energy.
5
- B. Ionic Bonding
- 1. Ionic bonds form when electrons are
transferred from one atom to another. 2.
Losing or gaining electrons, atoms participating
in ionic reactions fill outer shells, and are
more stable. 3. Example sodium with one
less electron has positive charge chlorine has
extra electron that has negative
charge. Such charged particles are called ions.
4. Attraction of oppositely charged ions
holds the two atoms together in an ionic bond.
6
- C. Covalent Bonding
- 1. Covalent bond results when two atoms
share electrons so each atom has octet of
electrons in outer shell. 2. Hydrogen
can give up electron to become hydrogen ion (H)
or share with another atom to complete its
outer shell of two electrons.
3. Structural formulas represent shared
atoms as a line between two atoms e.g., single
covalent bond (H-H), double covalent
bond (OO), and triple covalent bond (N three
lines N). 4. Three dimensional shape of
molecules is not represented by structural
formulas but is critical in understanding
the biological action of molecules
action of insulin, HIV receptors, etc.
7
- D. Nonpolar and Polar Covalent Bonds
- 1. In nonpolar covalent bonds, sharing of
electrons is equal. 2. With polar
covalent bonds, the sharing of electrons is
unequal. a. In water molecule (H2O),
sharing of electrons by oxygen and hydrogen is
not equal the oxygen atom with
more protons dominates the H2O association.
b. Attraction of an atom for
electrons in a covalent bond is called
electronegativity an oxygen atom is more
electronegative than hydrogen
atom. c. Oxygen in water molecule,
more attracted to electron pair, assumes small
negative charge. 3. Hydrogen Bonding
a. Hydrogen bond is weak attractive
force between slightly positive hydrogen atom of
one molecule and slightly
negative atom in another or the same molecule.
b. Many hydrogen bonds taken
together are relatively strong. c.
Hydrogen bonds between complex molecules of cells
help maintain structure and function.
8
- B. Properties of Water
- 1. The temperature of liquid water rises
and falls more slowly than that of most other
liquids. a. Calorie is amount of
heat energy required to raise temperature of one
gram of water 1 degree C. b. Because
water holds heat, its temperature falls more
slowly than other liquids this protects
organisms from rapid temperature
changes and helps them maintain normal
temperatures. 2. Water has a high heat
of vaporization. a. Hydrogen bonds
between water molecules require a large amount of
heat to break. b. This property
moderates earth's surface temperature permits
living systems to exist here. c.
When animals sweat, evaporation of the sweat
takes away body heat, thus cooling the animal.
3. Water is universal solvent,
facilitates chemical reactions both outside of
and within living systems. a. Water
is a universal solvent because it dissolves a
great number of solutes. b. Ionized
or polar molecules attracted to water are
hydrophilic. c. Nonionized and
nonpolar molecules that cannot attract water are
hydrophobic. 4. Water molecules are
cohesive and adhesive. a. Cohesion
allows water to flow freely without molecules
separating, due to hydrogen bonding.
b. Adhesion is ability to adhere to polar
surfaces water molecules have positive, negative
poles. c. Water rises up tree from
roots to leaves through small tubes.
1) Adhesion of water to walls of
vessels prevents water column from breaking
apart. 2) Cohesion allows
evaporation from leaves to pull water column from
roots. 5. Water has a high surface
tension measured by how difficult it is to break
the surface of a liquid. a. As with
cohesion, hydrogen bonding causes water to have
high surface tension. b. Permits a
rock to be skipped across pond surface supports
insect walking on water surface. 6.
Unlike most substances, frozen water is less
dense than liquid water. a. Below 4
degrees C, hydrogen boding becomes more rigid but
more open, causing expansion. b.
Because ice is less dense, it floats therefore
bodies of water freeze from the top down.
c. If ice was heavier than water,
ice would sink and ponds would freeze solid.
9
- . Acids and Bases
- 1. Covalently bonded water molecules
ionize the atoms dissociate into ions.
2. When water ionizes or dissociates, it releases
a small but equal number of H and OH- ions
thus its pH is neutral. 3.
Water dissociates into hydrogen and hydroxide
ions H - O - H H OH-. 4. Acid
molecules dissociate in water, releasing hydrogen
ions (H) ions HCl H Cl-. 5.
Bases are molecules that take up hydrogen ions or
release hyroxide ions. NaOH Na OH-.
6. The pH scale indicates acidity and basicity
(alkilinity) of a solution. a.
Measure of free hydrogen ions as a negative
logarithm of the H concentration (-log H).
b. PH values range from 0 most
acidic to 14 most basic. 1) One
mole of water has 10 to the 7 moles/liter of
hydrogen ions therefore, has neutral pH of 7.
2) Acid is a substance with pH
less than 7 base is a substance with pH greater
than 7. 3) As logarithmic scale,
each lower unit has 10 times the amount of
hydrogen ions as next higher pH unit
as move up pH scale, each
unit has 10 times the basicity of previous unit.
7. Buffers keep pH steady and within
normal limits in living organisms.
a. Buffers stabilize pH of a solution by taking
up excess hydrogen or hydroxide ions.
b. Carbonic acid helps keep blood pH
within normal limits H2CO3 H HCO3-.
10
- IV. Organic Molecules
- A. Definitions
- 1. Most common elements in living things
are carbon, hydrogen, nitrogen, and oxygen.
2. These four elements constitute about
95 of your body weight. 3. Chemistry of
carbon allows the formation of an enormous
variety of organic molecules. 4. Organic
molecules have carbon bonded to other atoms and
determine structure and function of living
things. 5. Inorganic molecules do not
contain carbon and hydrogen together inorganic
molecules (e.g., NaCl) can play
important roles in living things.
11
- B. Carbon Skeletons and Functional Groups
- 1. Carbon has four electrons in outer
shell bonds with up to four other atoms (usually
H, O, N, or another C). 2. Ability of
carbon to bond to itself makes possible carbon
chains and rings these structures serve as the
backbones of organic molecules. 3.
Functional groups are clusters of atoms with
characteristic structure and functions.
a. Polar molecules (with /-
charges) are attracted to water molecules and are
hydrophilic. b. Nonpolar molecules
are repelled by water and do not dissolve in
water these are hydrophobic. c.
Hydrocarbon is hydrophobic except when it has an
attached ionized functional group such as
carboxyl (acid) (--COOH) then
molecule is hydrophilic. d. Cells
are 70-90 water the degree organic molecules
interact with water affects their function.
4. Isomers are molecules with identical
molecular formulas but differ in arrangement of
their atoms (e.g.,
glyceraldehyde and dihydroxyacetone).
12
- C. Building Polymers
- 1. Four classes of polymers
(polysaccharides, triglycerides, polypeptides,
and nucleic acids) provide great
diversity. 2. Small organic molecules
(e.g., monosaccharides, glycerol and fatty acid,
amino acids, and nucleotides) serve
as monomers, the subunits of polymers.
13
- D. Condensation and Hydrolysis
- 1. Polymers are the large macromolecules
composed of three to millions of monomer
subunits. 2. Polymers build by different
bonding of different monomers mechanism of
joining and breaking these bonds is
condensation and hydrolysis. 3. Cellular
enzymes carry out condensation and hydrolysis of
polymers. 4. During condensation
synthesis, a water is removed (condensation) and
a bond is made (synthesis). a. When
two monomers join, a hydroxyl (--OH) group is
removed from one monomer and a hydrogen
is removed from the other.
b. This produces the water given off
during a condensation reaction. 5.
Hydrolysis reactions break down polymers in
reverse of condensation a hydroxyl (--OH) group
from water attaches to one monomer
and hydrogen (--H) attaches to the other.
14
- V. Carbohydrates
- A. Monosaccharides and Disaccharides
- 1. Monosaccharides are simple sugars with
a carbon backbone of three to seven carbon atoms.
a. Best known sugars have six
carbons (hexoses). 1) Glucose
and fructose isomers have same formula (C6H12O6)
but differ in structure. 2)
Glucose is commonly found in blood of animals is
immediate energy source to cells.
3) Fructose is commonly found in
fruit. 4) Shape of molecules is
very important in determining how they interact
with one another. 2. Ribose and
deoxyribose are five-carbon sugars (pentoses)
contribute to the backbones of RNA
and DNA respectively. 3. Disaccharides
contain two monosaccharides joined by
condensation. a. Lactose is composed
of galactose and glucose and is found in milk.
b. Maltose is two glucose molecules
forms in digestive tract of humans during starch
digestion. c. Sucrose is composed of
glucose and fructose and is transported within
plants.
15
- B. Polysaccharides are chains of glucose
molecules or modified glucose molecules (chitin).
- 1. Starch is straight chain of glucose
molecules with few side branches. 2.
Glycogen is highly branched polymer of glucose
with many side branches called "animal starch,"
it is storage carbohydrate of
animals. 3. Cellulose is glucose bonded
to form microfibrils primary constituent of
plant cell walls. a. Cotton is
nearly pure cellulose. b. Cellulose
is not easily digested due to the strong linkage
between glucose molecules. c.
Grazing animals can digest cellulose due to
special stomachs and bacteria. 4. Chitin
is polymer of glucose with amino acid attached to
each it is primary constituent of crabs and
related animals like lobsters and
insects.
16
(No Transcript)
17
(No Transcript)
18
Construct linear glucose
19
(No Transcript)
20
Good morning!Go to lab tables, construct one
linear glucose and one linear fructose per lab
table. What is an isomer?
21
Convert to ring glucose bya. Disaasembling the
double bond to oxygen on 1Cb. Take the hydroxide
(-OH) group off 5Cc. Bond the O that was
double bonded to 5Cd. Attach the OH group to 1C
22
Construct ring fructosea. Detach the double
bond from the O of 2Cb. Detach the OH from
5Cc. Bond the O to 5Cd. Attach OH group to
opening in 2C
23
5. Make sucrose Condensation Reactiona. Orient
molecules as seen belowb. Remove OH group from
1C on a-glucosec. Remove H from hydroxide group
of 5C on fructosed. Bond O to 1C of glucose,
H2O is released
24
(No Transcript)
25
- VI. Lipids
- A. Lipids are varied in structure.
- 1. Many are insoluble in water because
they lack polar groups. 2. Fat provides
insulation and energy storage. 3.
Phospholipids from plasma membranes and steroids
are important cell messengers
26
- B. Fats and Oils
- 1. A fatty acid is a long hydrocarbon
chain with a carboxyl (acid) group at one end.
a. Because the carboxyl group is a
polar group, fatty acids are soluble in water.
b. Most fatty acids in cells contain
16 to 18 carbon atoms per molecule.
c. Saturated fatty acids have no double bonds
between their carbon atoms. d.
Unsaturated fatty acids have double bonds in the
carbon chain where there are less than
two hydrogens per carbon atom.
e. Saturated animal fats are
associated with circulatory disorders plant oils
can be substituted for animal
fats in the diet. 2. Glycerol is a
water-soluble compound with three hydroxyl
groups. 3. Triglycerides are glycerol
joined to three fatty acids by condensation.
4. Fats are triglycerides containing
saturated fatty acids (e.g., butter is solid at
room temperature). 5. Oils are
triglycerides with unsaturated fatty acids (e.g.,
corn oil is liquid at room temperature).
6. Animals use fat rather than glycogen for
long-term energy storage fat stores more energy
27
- C. Waxes
- 1. Waxes are a long-chain fatty acid
bonded to a long-chain alcohol. 2. Solid
at room temperature, waxes have a high melting
point and are waterproof and resist degradation.
3. Waxes form a protective covering that
retards water loss in plants, and maintains
animal skin and fur.
28
- D. Phospholipids
- 1. Phospholipids are like neutral fats
except one fatty acid is replaced by phosphate
group or a group with both phosphate
and nitrogen. 2. Phosphate group is the
polar head hydrocarbon chains become nonpolar
tails. 3. Phospholipids arrange
themselves in a double layer in water, so the
polar heads face outward toward
water molecules and nonpolar tails face toward
each other away from water molecules. 4.
This property enables them to form an interface
or separation between two solution (e.g., the
interior and exterior of a cell)
the plasma membrane is a phospholipid bilayer.
29
- E. Steroids
- 1. Steroids differ from neutral fats
steroids have a backbone of four fused carbon
rings vary according to attached
functional groups. 2. Functions vary due
primarily to different attached functional
groups. 3. Cholesterol is a part of an
animal cells membrane and a precursor of other
steroids, including aldosterone and
sex hormones. 4. Testosterone is the
male sex hormone.
30
At each lab table, construct three fatty acids
and one glycerol molecule
31
2. Combine to form a triglyceride
32
(No Transcript)
33
(No Transcript)
34
- VII. Proteins
- A. Protein Functions
- 1. Support proteins include keratin,
which makes up hair and nails, and
collagenfibers, which support many organs.
2. Enzymes are proteins that act as
organic catalysts to speed chemical reactions
within cells. 3. Transport functions
include channel and carrier proteins in the
plasma membrane and hemoglobin that
carries oxygen in red blood cells. 4.
Defense functions include antibodies that prevent
infection. - 5. Hormones include insulin that
regulates glucose content of blood. 6.
Motion is provided by myosin and actin proteins
that make up the bulk of muscle.
35
- B. Amino Acids
- 1. All amino acids contain an acidic
group (---COOH) and an amino group (--NH2).
2. Amino acids differ in nature of R
group, ranging from single hydrogen to
complicated ring compounds. 3. R group
of amino acid cysteine ends with a sulfhydryl
(--SH) that serves to connect one chain of amino
acids to another by a disulfide bond
(--SS). 4. There are 20 different amino
acids commonly found in cells.
36
- C. Peptides
- 1. Peptide bond is a covalent bond
between amino acids in a peptide. 2.
Atoms of a peptide bond share electrons unevenly
(oxygen is more electronegative than nitrogen).
3. Polarity of the peptide bond permits
hydrogen bonding between parts of a polypeptide.
4. A peptide is two or more amino acids
joined together. 5. Polypeptides are
chains of many amino acids joined by peptide
bonds. a. Protein may contain more
than one polypeptide chain it can have large
numbers of amino acids.
37
- D. Levels of Protein Structure
- 1. Shape of a protein determines function
of the protein in the organism. 2.
Primary structure is sequence of amino acids
joined by peptide bonds. a.
Frederick Sanger determined first protein
sequence, with hormone insulin, in 1953.
b. First broke insulin into
fragments and determined amino acid sequence of
fragments. c. Then determined
sequence of the fragments themselves.
d. Required ten years research
modern automated sequencers analyze sequences in
hours. e. Since amino acids differ
by R group, proteins differ by a particular
sequence of the R groups. 3. Secondary
structure results when a polypeptide takes a
particular shape. a. The alpha helix
was the first pattern discovered by Linus Pauling
and Robert Corey. 1) In peptide
bonds oxygen is partially negative, hydrogen is
partially positive. 2) This
allows hydrogen bonding between the CO of one
amino acid and the NH of another.
3) Hydrogen bonding between
every fourth amino acid holds spiral shape of an
alpha helix. 4) Alpha helices
covalently bonded by disulfide (--SS--) linkages
between two cysteine amino acids. b.
The beta sheet was the second pattern discovered.
1) Pleated beta sheet
polypeptides turn back upon themselves hydrogen
bonding occurs between
extended lengths. 2)
Beta-keratin includes keratin of feathers,
hooves, claws, beaks, scales, and horns silk
also is protein with beta
sheet secondary structure. 4. Tertiary
structure results when proteins of secondary
structure are folded, due to various interactions
between the R groups of their
constituent amino acids. 5. Quaternary
structure results when two or more polypeptides
combine. a. Hemoglobin is globular
protein with a quaternary structure of four
polypeptides. b. Most enzymes have a
quaternary structure.
38
- E. Denaturation of Proteins
- 1. Both temperature and pH can change
polypeptide shape. a. Examples
heating egg white causes albumin to congeal
adding acid to milk causes curdling.
b. When such proteins lose their normal
configuration, the protein is denatured.
c. Once a protein loses its normal
shape, it cannot perform its usual function.
2. The sequence of amino acids therefore
causes the proteins final shape.
39
(No Transcript)
40
Construct an amino acid
41
- VIII. Nucleic Acids
- A. Nucleic Acid Functions
- 1. Nucleic acids are huge polymers of
nucleotides with very specific functions in
cells. 2. DNA (deoxyribonucleic acid) is
the nucleic acid whose nucleotide sequence stores
the genetic code for its own
replication and for the sequence of amino acids
in proteins. 3. RNA (ribonucleic acid)
is a single-stranded nucleic acid that translates
the genetic code of DNA into the
amino acid sequence of proteins 4.
Nucleotides have metabolic functions in cells.
a. Coenzymes are molecules which
facilitate enzymatic reactions. b.
ATP (adenosine triphosphate) is a nucleotide used
to supply energy. c. Nucleotides
also serve as nucleic acid monomers.
42
(No Transcript)
43
(No Transcript)
44
45
46
(No Transcript)
47
(No Transcript)
48
(No Transcript)
49
(No Transcript)