MATTER CYCLING IN ECOSYSTEMS - PowerPoint PPT Presentation
1 / 35
Title:
MATTER CYCLING IN ECOSYSTEMS
Description:
Mechanical energy (moving, thinking, living) Chemical energy (photosynthesis) Chemical energy (food) Solar energy Waste Heat Waste Heat Waste Heat Waste Heat Fig. 2 ... – PowerPoint PPT presentation
Number of Views:186
Avg rating:3.0/5.0
Title: MATTER CYCLING IN ECOSYSTEMS
1
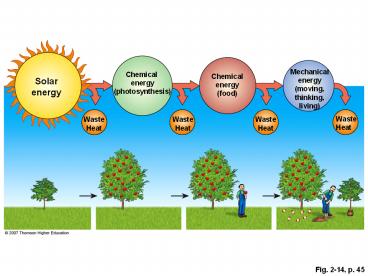
Mechanicalenergy(moving,thinking,living)
Chemical energy (photosynthesis)
Chemical energy (food)
Solar energy
Waste Heat
Waste Heat
Waste Heat
Waste Heat
Fig. 2-14, p. 45
2
SUSTAINABILITY AND MATTER AND ENERGY LAWS
- Unsustainable High-Throughput Economies Working
in Straight Lines - Converts resources to goods in a manner that
promotes waste and pollution.
Figure 2-15
3
Sustainable Low-Throughput Economies Learning
from Nature
- Matter-Recycling-and-Reuse Economies Working in
Circles - Mimics nature by recycling and reusing, thus
reducing pollutants and waste. - It is not sustainable for growing populations.
4
Inputs (from environment)
System Throughputs
Outputs (into environment)
Energy conservation
Low-quality Energy (heat)
Energy
Sustainable low-waste economy
Waste and pollution
Waste and pollution
Pollution control
Matter
Recycle and reuse
Matter Feedback
Energy Feedback
Fig. 2-16, p. 47
5
Biogeochemical Cycling
- The cycling of nutrients through ecosystems via
food chains and food webs, including the exchange
of nutrients between the biosphere and the
hydrosphere, atmosphere and geosphere (e.g.,
soils and sediments)
6
MATTER CYCLING IN ECOSYSTEMS
- Nutrient Cycles Global Recycling
- Global Cycles recycle nutrients through the
earths air, land, water, and living organisms. - Nutrients are the elements and compounds that
organisms need to live, grow, and reproduce. - Biogeochemical cycles move these substances
through air, water, soil, rock and living
organisms.
7
Transfer v Transformation
- Transfers flow through a system and involve a
change in location - Transformations lead to interaction within a
system in the formation of a new end product or
involving a change of state. - As we discuss various cycles, underline
transfers, and circle transformations.
8
Flows v Storage
- Sometimes matter flows through a cycle and
sometimes it is stored. - When a material is flowing through a cycle
(conversion), color it green. - When it is being stored (sink), color it red.
9
Nutrient cycles and energy flow
10
The Water Cycle
Figure 3-26
11
Waters Unique Properties
- There are strong forces of attraction between
molecules of water. - Water exists as a liquid over a wide temperature
range. - Liquid water changes temperature slowly.
- It takes a large amount of energy for water to
evaporate. - Liquid water can dissolve a variety of compounds.
- Water expands when it freezes.
12
Effects of Human Activities on Water Cycle
- We alter the water cycle by
- Withdrawing large amounts of freshwater.
- Clearing vegetation and eroding soils.
- Polluting surface and underground water.
- Contributing to climate change.
13
The global carbon cycle
14
The Carbon CyclePart of Natures Thermostat
Figure 3-27
15
Carbon
- Photosynthesis and Respiration provide a link
between the atmosphere and terrestrial - environments.
- Decomposition recycles carbon to the soil and
back to atmosphere - Fires oxidize organic material to CO2 (burning)
- Organic detritus, under intense pressure,
changes into coal and petroleum in rock. - Limestone keeps carbon out of circulation
- Weathering of exposed limestone releases carbon
- A carbon atom cycles about every six years
- The basic constituent of all organic compounds
16
Human impacts on the carbon cycle
- Human intrusion into the cycle is significant
- We are diverting or removing 40 of the
photosynthetic effect of land plants - Burning fossil fuels has increased atmospheric
CO2 by 35 - Deforestation and soil degradation release
significant amounts of CO2 to the atmosphere - Recent reforestation and changed agricultural
practices have improved this somewhat
17
The Nitrogen Cycle Bacteria in Action
Figure 3-29
18
The global nitrogen cycle
19
Major Components of Nitrogen Cycle
20
(No Transcript)
21
The nitrogen cycle
- Is a unique cycle
- Bacteria in soils, water, and sediments perform
many steps of the cycle - Nitrogen is in high demand by aquatic and
terrestrial plants - Air is the main reservoir of nitrogen (N)
- Nonreactive nitrogen most organisms can not use
it - Reactive nitrogen (Nr) other forms of nitrogen
that can be used by organisms
22
Plants take up nitrogen
- Plants in terrestrial ecosystems (non-N-fixing
producers) - Take up Nr as ammonium (NO4) and incorporate it
into proteins and nucleic acid compounds - The nitrogen moves through the food chain to
decomposers, releasing nitrogen wastes - Soil bacteria (nitrifying bacteria) convert
ammonium to nitrate to obtain energy - Nitrate is available for plant uptake
- Nitrogen fixation bacteria and cyanobacteria can
use nonreactive N and convert it to a usable form
23
The Nitrogen Cycle
- Nitrogen Fixation
- Bacteria convert gaseous nitrogen to ammonia
- (N2) (NH3)
- Some ammonia enters the ground normally through
waste and decay as well (pee, poop and dead
things).
Different bacteria convert ammonia to
nitrite (NH3) (NO2-)
Bacteria use nitrite as an energy source, and
give off nitrate (NO3-) as waste Nitrate is
then taken up by plants or released into the
atmosphere, where it becomes gaseous N2 again.
(NO3-)
(N2)
24
Nitrogen fixation
25
Effects of Human Activities on the Nitrogen Cycle
- We alter the nitrogen cycle by
- Adding gases that contribute to acid rain.
- Adding nitrous oxide to the atmosphere through
farming practices which can warm the atmosphere
and deplete ozone. - Contaminating ground water
- from nitrate ions in inorganic
- fertilizers.
- Releasing nitrogen into the
- troposphere through
- deforestation.
26
(No Transcript)
27
The global phosphorus cycle
28
The Phosphorous Cycle
Figure 3-31
29
The phosphorus cycle
- Mineral elements originate in rock and soil
minerals - A shortage of phosphorus is a limiting factor
- Excessive phosphorus can stimulate algal growth
- As rock breaks down, phosphate is released
- Replenishes phosphate lost through leaching or
runoff - Organic phosphate incorporated into organic
compounds by plants from soil or water
30
Human impacts on the phosphorus cycle
- The most serious intrusion comes from fertilizers
- Phosphorus is mined and made into fertilizers,
animal feeds, detergents, etc. - When added to soil, it can stimulate production
- Human applications have tripled the amount
reaching the oceans, accelerating the cycle - It cant be returned to the soil
- Excess phosphorus in water leads to severe
pollution - Can cause too many bacteria and fish kills
31
The Sulfur Cycle
Figure 3-32
32
Why sulfur?
- -In nature, it can be found as the pure element
and as sulfide and sulfate minerals. - -It is an essential element for life and is found
in two amino acids cysteine and methionine. - -Its commercial uses are primarily in
fertilizers, but it is also widely used in black
gunpowder, matches, insecticides and fungicides.
33
Effects of Human Activities on the Sulfur Cycle
- We add sulfur dioxide to the atmosphere by
- Burning coal and oil
- Refining sulfur containing petroleum.
- Convert sulfur-containing metallic ores into free
metals such as copper, lead, and zinc releasing
sulfur dioxide into the environment.
34
Serious consequences of fertilization
- Nitric acid has destroyed lakes, ponds, and
forests - Atmospheric nitrogen oxides adds to ozone
pollution, climate change, and stratospheric
ozone depletion - Abundant nitrates are not incorporated into
organisms - They are released into the soil, where they leach
calcium and magnesium - Eutrophication of waterways
- Nitrogen cascade complex of ecological effects
as Nr moves through the environment
35
Comparing the cycles
- Carbon is mainly found in the atmosphere
- Directly taken in by plants
- Nitrogen and phosphorus are limiting factors
- All three cycles have been sped up by human
actions - Acid rain, greenhouse gases, eutrophication
- Other cycles exist for other elements (e.g.,
water) - All go on simultaneously
- All come together in tissues of living things