Lipases - PowerPoint PPT Presentation
1 / 21
Title:
Lipases
Description:
Nu dus een paar dia's.... Rhizomucor miehei lipase in the closed form: ... Metal-dependent (Zn2 cofactor) Aldolases from (hyper)thermophilic microorganisms ... – PowerPoint PPT presentation
Number of Views:1148
Avg rating:3.0/5.0
Title: Lipases
1
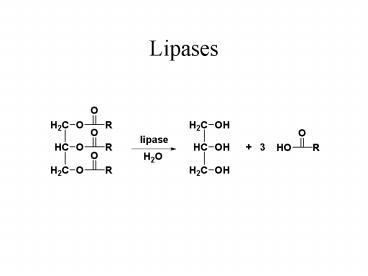
Lipases
2
native enzyme
R3-OH
Bi-bi ping-pong mechanism !
R1-OH
3
(No Transcript)
4
Esterolytic vs. lipolytic activity
Esterase activity
Lipase activity
5
5
3
3
2
2
Activity
Activity
1
1
0
0
1
0
2
3
1
0
2
3
Substrate concentration
Substrate concentration
5
Nu dus een paar dia's....
6
Rhizomucor miehei lipase in the closed form
nucleophilic elbow green, lid blue-green
7
Candida antarctica lipase B (no lid)
nucleophilic elbow green (centre)
8
Current lipase research _at_ OC
9
Epoxide hydrolase from yeasts
10
Reaction mechanism of epoxide hydrolase
11
Present research on epoxide hydrolase
Resolution of
as chiral building blocks for pharmaceuticals
More info dr. Carel Weijers!
12
Development and integration of stable aldolases
in the synthesis of enantiopure functionalized
nitrogen heterocyclesMaud CABRIÈRES, Maurice
FRANSSEN
13
IBOS PROJECT 5 partners
- IBOS Integration of Biosynthesis and Organic
Synthesis Program - Dr. Franssen/Prof. Sudhölter (Org. Chem. WU)
- Dr. van der Oost/Prof. de Vos (Microbiology WU)
- Prof. Kieboom, University of Leiden (Bio-org.
Chem. UL) - Prof. Rutjes, University of Nijmegen (Org. Chem.
KUN) - Industrial partner DSM
14
General framework of IBOS project
- Schematic overview
- of the project
- Final goal integration of new generation of
Aldolases in a cascade of biocatalytic
conversions, aiming at the synthesis of novel
nitrogen heterocyclic compounds
Aldehyde
Hydroxynitrile Lyase (HNL)
Cyanohydrin
15
C-C bond formation relevance
- Carbon- carbon bond formation lies at the heart
of many organic synthesis - Todays fine chemistry challenge forming
building blocks with complete control of the
stereochemistry of stereogenic centres - Aldol reaction one of the most powerful and
famous method to enlarge carbon skeleton
16
Aldol condensation reaction
- Racemic mixture!
17
Aldol condensation Nature uses aldolases
- Specific group of lyases, very stereoselective
- Nearly 30 natural aldolases identified (2000)
- Broad range of acceptors
- Stringent requirement for donors
18
Aldolase classification based on catalytic
mechanism
- Type II Aldolase
- Donor activation
- Metal-dependent (Zn2 cofactor)
- Type I Aldolase
- Donor activation
- Imine intermediate
19
Aldolases from (hyper)thermophilic microorganisms
- Archaea and bacteria with Toptimum gt 80 C
- Deep sea smokers Pyrococcus
- Shallow marine vents Thermotoga (DHPS)
- Acidic hot spring Sulfolobus (KDG)
20
Relevant Aldolases involved in this project
- KDG (2-keto-3-deoxy gluconate aldolase)
- DHPS (2,3-dihydrodipicolinate synthase)
- DERA (2-deoxyribose-5-phosphate aldolase)
21
More information on what we do
http//www.ftns.wau.nl/oc/research/biocatalysis/bi
ocatalysis.htm