Periodic Table PowerPoint PPT Presentation
1 / 50
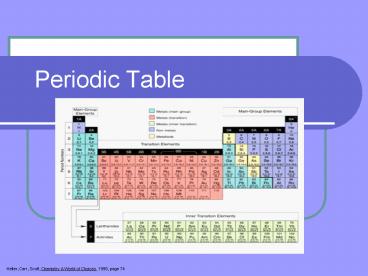
Title: Periodic Table
1
Periodic Table
Kelter, Carr, Scott, Chemistry A World of Choices
1999, page 74
2
History of the Periodic Table
3
Dmitri Mendeleev
- Russian - 1872
- Invented periodic table
- Organized elements by properties
- Arranged elements by atomic mass
- Predicted existence of several unknown elements
- Element 101
Dmitri Mendeleev
4
Dmitri Mendeleyev (1834-1907)
- observed that elements listed in order of atomic
mass showed regularly (or periodically) repeating
properties. - He announced his Periodic Law in 1869 and
published a list of known elements in a tabular
form. - He had the courage to leave gaps where the
Periodic Law did not seen to fit, predicting that
new elements would be discovered to fill them.
5
Modern Periodic Table
- H.G.J. Moseley
- Arranged elements by increasing atomic number
- Killed in WW I at age 28
- (Battle of Gallipoli)
1887 - 1915
6
Periodic Law
- When elements are arranged in order of
increasing atomic number, their physical and
chemical properties show a PERIODIC pattern
7
Organization of the Table
- Elements that have similar properties are aligned
in vertical columns called GROUPS or FAMILIES. - Elements of increasing atomic numbers are
arranged in horizontal rows called PERIODS.
8
Groups of Elements
9
Groups
- All elements in a group have the same number of
valence electrons (outermost) - All elements in a group have similar chemical
properties - Increase in the metallic properties from top to
bottom
10
- Group 1 Alkali Metals
- Metallic Properties
- Tarnish rapidly
- Easily forms 1 ions
- Soft enough to cut with a knife
- EXTREMELY reactive (air)
- Valence configuration of s1
- Group 2 - Alkaline Earth
- Metallic properties
- Form 2 ions
- Higher densities and melting points of group 1
- Valence configuration of s2
11
Potassium Metal in Water
Newmark, CHEMISTRY, 1993, page 25
12
- Group 17 (VIIA)
- Halogens
- Very Reactive
- Various Ions but usually -1
- Valence configuration is s2p5
- Group 18 (VIIIA)
- Noble Gases
- Very UNreactive
- Called the inert, rare gases, monatomic gases
- Valence configuration is s2p6
13
Transition Elements
- D-block elements
- Have several empty or half filled d orbitals
- Multiple oxidation states because the d orbital
values are close to those of s orbital values and
different electrons can be removed - Form colored solutions
- Most have high density and high melting points
14
Metals, Nonmetals, Metalloids
15
Metals and Nonmetals
He 2
H 1
1
Li 3
C 6
N 7
O 8
F 9
Ne 10
B 5
Be 4
Nonmetals
2
Na 11
Al 13
Si 14
P 15
S 16
Cl 17
Ar 18
Mg 12
3
K 19
Ca 20
Sc 21
Ti 22
V 23
Cr 24
Mn 25
Fe 26
Co 27
Ni 28
Cu 29
Zn 30
Ga 31
Ge 32
As 33
Se 34
Br 35
Kr 36
4
METALS
Rb 37
Sr 38
Y 39
Zr 40
Nb 41
Mo 42
Tc 43
Ru 44
Rh 45
Pd 46
Ag 47
Cd 48
In 49
Sn 50
Sb 51
Te 52
I 53
Xe 54
5
Cs 55
Ba 56
Hf 72
Ta 73
W 74
Re 75
Os 76
Ir 77
Pt 78
Au 79
Hg 80
Tl 81
Pb 82
Bi 83
Po 84
At 85
Rn 86
6
Fr 87
Ra 88
Rf 104
Db 105
Sg 106
Bh 107
Hs 108
Mt 109
7
W
Ce 58
Pr 59
Nd 60
Pm 61
Sm 62
Eu 63
Gd 64
Tb 65
Dy 66
Ho 67
Er 68
Tm 69
Yb 70
Lu 71
La 57
Th 90
Pa 91
U 92
Np 93
Pu 94
Am 95
Cm 96
Bk 97
Cf 98
Es 99
Fm 100
Md 101
No 102
Lr 103
Ac 89
16
Metals Vs Non-Metals
- 2/3 of all elements
- Have luster
- Good conductors of heat and electricity
- Solid at room temp. (except Hg)
- Malleable
- Ductile
- Tend to LOSE electrons
- Poor conductors of heat and electricity
(insulators) - No luster
- Not malleable or ductile (brittle)
- Gaseous, liquid or solid
- Tend to gain or share electrons.
17
Semimetals/metalloid
- On the stepline
- Could have a combination of metal and nonmetal
properties - Considered the natural change in character of
elements as you go across the period - Only 7 elements
18
metallic character increases
nonmetallic character increases
metallic character increases
nonmetallic character increases
19
Solids, Liquids, and Gases
- Most elements are solids.
- Two liquids on the periodic table Mercury (Hg)
is a metal and Bromine (Br) is a nonmetal. - Ten gases on the periodic table H2, He, N2, O2,
F2, Cl2, Ne, Ar, Kr, Xe, Rn
20
Diatomic Molecules
BrINCLHOF twins
H2 O2 Br2 F2 I2 N2 Cl2
21
Periodic Trends
22
Ionization Energy
- The energy needed to remove one of an atoms
electrons. - The greater the shielding effect the easier to
remove an electron.
23
Shielding Effect
Valence
-
-
nucleus
-
Electrons
-
Electron Shield kernel electrons
- Kernel electrons block the attractive force of
the nucleus from the valence electrons
24
First Ionization energy
Atomic number
25
Electronegativity
- the ability to attraction electrons in a bond
- based on 0-4 scale
- Which element has the highest? the lowest?
- Fluorine and Francium
26
(No Transcript)
27
Electronegativity in a Group
28
Explanation of the trend
- Electronegativity decreases down a group.
- atomic radius increases
- outer electrons are shielded from the attraction
of the nucleus - bonding electrons are less strongly attracted to
nucleus
29
Electronegativity in a Period
30
Explanation of the Trend
- Electronegativity increases across a period
- nuclear charge increases
- atomic radius decreases
- shielding is negligible because same energy level
- bonding electrons more strongly attracted to the
nucleus
31
Atomic Radius
- The distance from the center of the atoms
nucleus to the outer edge of the outermost
electron.
32
Periodic Trends in Atomic Radii
LeMay Jr, Beall, Robblee, Brower, Chemistry
Connections to Our Changing World , 1996, page
175
33
Atomic Radius - Summary
- Across a period, radius decreases because there
is a greater pull on the electrons from the
nucleus. - Down a period, radius increases because
additional energy level is added.
34
Relative Size of Atoms
Zumdahl, Zumdahl, DeCoste, World of Chemistry
2002, page 350
35
Ionic Size
- Cations form by losing electrons.
- Cations are smaller than the atom they come from.
- Metals form cations.
- Cations of representative elements have noble gas
configuration.
36
Energy
e
e
Li
Li
e
Lithium ion
Lithium atom
37
Ionic size
- Anions form by gaining electrons.
- Anions are bigger that the atom they come from.
- Nonmetals form anions.
- Anions of representative elements have noble gas
configuration.
38
Trends in Atomic and Ionic Size
Metals
Nonmetals
Group 1A
Group 3A
F-
F
64
60
136
Cl-
Cl
99
181
Br-
Br
114
195
Cations are smaller than parent atoms
Anions are larger than parent atoms
39
Summary of Periodic Trends
Shielding is constant Atomic radius
decreases Ionization energy increases Electronegat
ivity increases Nuclear charge increases
1A
0
Nuclear charge increases Shielding
increases Atomic radius increases Ionic size
increases Ionization energy decreases Electronegat
ivity decreases
2A
3A
4A
6A
7A
5A
Ionic size (cations) Ionic size
(anions) decreases decreases
40
Measurement
41
How to measure
- What is the size of the paper?
- How many decimal places should the answer have?
42
More Measurement
- How tall is the plant to the correct number of
decimal places?
43
Metric System
- k h da base d c - m
44
Accuracy vs. Precision
- Accuracy how close the measurement is to a
true value - Precision - how close several measurements are
to each other
45
Significant Figures
- The A P rule
- Decimal absent Atlantic Side and count across
the country. - Decimal present Pacific Side and count across
the country.
46
Significant Figures Practice
- How many sig figs present?
- 37400
- 300.0
- 0.0045
- Round each to two significant digits.
- 349987
- 0.3445
47
Significant Figures and Calculations
- For and - reduce answer to least number of
decimal places in the problems - For x and ? reduce answer to least number of
significant figures in the problem.
48
Sig Fig Calculation Practice
- 345.1 27. 35
- 99.456 - 34
- 435.2 x 76.1
- 150 / 4
49
Density
- ratio of mass to volume
- D m/v on reference table T
- density is not size dependent.
- What is the density of a 3 cm cube that
- weighs 27 g?
50
Percent Error
- used to analyze lab data
- closeness the true value
- ?observed accepted ? x 100 on table T
- accepted
- If a student calculates the mass of Ne to be
19.9 g and the true value is 20.2 g what is the
percent error?