Figure 22'1 Neurulation in the mammalian embryo - PowerPoint PPT Presentation
1 / 21
Title:
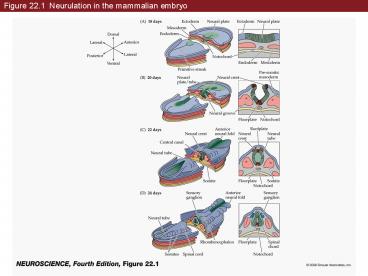
Figure 22'1 Neurulation in the mammalian embryo
Description:
Figure 22.3 Major inductive signaling pathways in vertebrate embryos ... Figure 22.8 Generation of cortical neurons during the gestation of a rhesus monkey ... – PowerPoint PPT presentation
Number of Views:690
Avg rating:3.0/5.0
Title: Figure 22'1 Neurulation in the mammalian embryo
1
Figure 22.1 Neurulation in the mammalian embryo
2
Figure 22.2 The neural crest
3
Figure 22.3 Major inductive signaling pathways
in vertebrate embryos
4
Box 22B Retinoic Acid Teratogen and Inductive
Signal
Mid-gestation mouse embryo
Normal action of retinoic acid
Following maternal ingestion of retinoic acid
Following maternal ingestion of retinoic acid
Normal action of retinoic acid
5
Figure 22.4 Local signals specify sensory relay
neurons, interneurons, and motor neurons (Part 2)
6
Figure 22.5 Regional specification of the
developing brain
7
Figure 22.7 Nuclear migration and mitosis in
precursor cells in vertebrate neuroepithelium
8
Figure 22.9 Mechanisms that guide neuronal and
glial differentiation in neural ectoderm
9
Figure 22.12 Radial migration in the developing
cortex
10
Figure 22.8 Generation of cortical neurons
during the gestation of a rhesus monkey
11
Figure 22.13 Mutations in genes that influence
neuronal migration cause malformations of the
human cerebral cortex
DCXDoublecortin
12
Box 22G Mixing It Up Long-Distance Neuronal
Migration
13
Variable contribution of radial and tangential
migration to assembly of the cerebral cortex
14
Figure 22.2 The neural crest
15
Figure 22.11 Gene expression and cellcell
signaling during neural crest development
16
Stage-specific control of neuronal migration by
somatostatin Elina Yacubova and Hitoshi
Komuro Nature 415, 77-81 (2002)
Developing neurons transiently express
somatostatin and its receptors, but little is
known about their function at these early stages.
As we thought that endogenous somatostatin might
control the migratory behaviour of immature
neurons, we have examined the effects of
somatostatin in cerebellar granule cells of early
postnatal mice. Here we show that somatostatin
has opposite and stage-specific effects on the
migration of cerebellar granule cells. Activation
of somatostatin receptors increases the rate of
granule cell migration near their birthplace, but
decreases the rate near their final destination.
These results indicate that somatostatin may
provide an essential cue for accelerating the
movement of granule cells in the early phase and
for terminating the movement in the late phase.
17
Background
Calcium transients regulate migration of
cerebellar granule cells
What generates these calcium transients?
Neurons transiently express somatostatin and
somatostatin receptors
The Approach
Study cerebellar slices from postnatal day 10 mice
Label granule cells with DiI (fluorescent dye)
Apply somatostatin at different points during
granule cell migration
Analyze expression of calcium transients
18
Figure 2
Role of somatostatin on granule cell migration in
different cerebellar cortical layers
7 µm
19
Figure 2 3
Effects of somatostatin agonists and antagonist
on granule cell migration
Agonists
20
Figure 4
Changes in intracellular Ca2 fluctuations in
response to somatostatin are correlated with
changes in migration rate in culture
One day in culture
Two days in culture
The Ca2 fluctuations and changes in migration
rate are blocked by BAPTA, a Ca2 chelator
21
Stage-specific control of neuronal migration by
somatostatin Elina Yacubova and Hitoshi
Komuro Nature 415, 77-81 (2002)
Developing neurons transiently express
somatostatin and its receptors, but little is
known about their function at these early stages.
As we thought that endogenous somatostatin might
control the migratory behaviour of immature
neurons, we have examined the effects of
somatostatin in cerebellar granule cells of early
postnatal mice. Here we show that somatostatin
has opposite and stage-specific effects on the
migration of cerebellar granule cells. Activation
of somatostatin receptors increases the rate of
granule cell migration near their birthplace, but
decreases the rate near their final destination.
These results indicate that somatostatin may
provide an essential cue for accelerating the
movement of granule cells in the early phase and
for terminating the movement in the late phase.
Conclusions 1) Somatostatin has opposite and
stage-specific effects on the migration of
cerebellar granule cells. 2) Somatostatin exerts
its effects by generating transient elevations of
intracellular Ca2. 3) The mechanism by which
Ca2 transients dynamically regulate migration
rate remains to be elucidated.