Symmetry, pointgroups PowerPoint PPT Presentation
1 / 28
Title: Symmetry, pointgroups
1
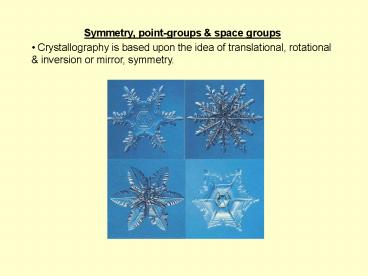
- Symmetry, point-groups space groups
- Crystallography is based upon the idea of
translational, rotational inversion or mirror,
symmetry.
2
Crystal definition A crystal is an object with
translational symmetry r(r) r(r) u a v
b w c
Has crystal symmetry
Doesnt have crystal symmetry
3
- Crystal Lattice
- A crystal has translational symmetry by
definition. - - If r (r) is the electron density within a
crystal at r then there exists vectors a, b c
such that - r (r) r (r u a v b w c)
- where u, v w are integers.
- Each identical copy is called a unit cell.
- a, b c are called unit cell vectors.
- Unit cell vector lengths are a a, b b, c
c. - a, b g describe the angles between unit cell
vectors. - Use a right handed coordinate system.
4
- Fractional coordinates.
- Any position within the crystal can be described
by - r (u x) a (v y) b (w z) c
- where u, v w are integers 0 lt x, y, z lt 1.
- x, y z are called fractional coordinates
describe a position within the unit cell.
5
- Lattice Planes
- Lattice planes are planes which pass through the
lattice points ? - Labled after the fractional position where they
first cross the a, b c axes. - If a lattice plane crosses the axes at the
fractional coordinates (x, y, z) then the lattice
plane is given the indices (h, k, l) equal to
(1/x, 1/y, 1/z).
6
- Other crystallographic symmetry
- In addition to translational symmetry there is
frequently other symmetry within the crystal
lattice - - Rotational symmetry.
- - Mirror plans.
- - Inversion.
- - Combinations of rotation or mirror planes plus
translation.
7
- Rotational symmetry.
- Twofold rotation (360o/2) written 2 or
- Threefold rotation (360o/3) written 3 or
- Fourfold rotation (360o/4) written 4 or
- Six-fold rotation (360o/6) written 6 or
- To have 4, two axes must have equal length one
angle 90o. - Also holds for 6 (or 3), but one angle must
be120o. - Not so restricted to have 2.
8
- Which rotations are consistent with a lattice?
- Suppose f 360o/n then (from figure)
- 2a cos f u a
- since it must be possible to get from one point
to the next by the lattice translation symmetry. - Only possible solutions are u -2, -1, 0, 1 or
2 only.
2 a cos f
f
Rotates to here
a
f
a
9
- Only 1, 2, 3, 4 6 rotations consistant with
translational symmetry. - Eg. 5 or 10 are never seen as crystal rotational
symmetries!
10
- Rotation matrix.
- r2 Rn r1.
- Eg. For rotational symmetry 2 about the y axis
- Hence (x, y, z) ? (-x, y, -z)
11
- Mirror planes
- The mirror plane normal is parallel with an axis
of the lattice. - If the mirror plane is parallel to z in a right
angle system - r2 Mz r1.
- where
- Hence (x, y, z) ? (x, y, -z).
c
b
a
12
- Inversion
- An inversion obeys the relation
- r2 - I r1.
- where
- Note I R2 M
13
- Screw Axes
- A screw axis is a translation by a fraction of
the unit cell followed by a rotation. - mn means translate n/m of the unit cell then
rotate 360o/m. - 21 means translate 1/2 of the unit cell rotate
180o - 31 means translate 1/3 of the unit cell rotate
120o. - 42 means translate 2/4 of the unit cell rotate
90o. - 41 means translate 1/4 of the unit cell rotate
90o.
c
eg. 42 about b
b
a
14
- Screw axes
- 21 drawn
- 31 drawn
- 42 drawn
- 63 drawn
- Red indicates translated ½ unit cell into the
page.
15
- Glide planes
- A glide plane means a translation followed by a
reflection in a plane (not certain how plane
defined). - a is a glide translation of ½ along a.
- b is a glide translation of ½ along b.
- c is a glide translation of ½ along c.
- n is a glide translation ½ along a face
diagional. - d is a glide translation ¼ along a face
diagional.
eg. b
Note need to check reflection correct!
16
- Primitive
crystal systems - Have 2, 3, 4 6 fold rotational symetry
possible. - At least two axes must be equal for 3, 4 or 6
fold rotational symmetry. - - These ideas underly the division into seven
primitive unit-cells.
Triclinic contains no extra symmetry. a ? b ?
c a ? b ? g ? 90o
Monoclinic contains one 2-fold rotation. a ? b
? c two of a or b or g 90o
17
Orthorhombic a ? b ? c a b g 90o Has
three 2-fold axes.
Tetragonal a b ? c a b g 90o Has a
4-fold axes.
Cubic a b c a b g 90o Has three
4-fold axes.
18
Trigonal Rhombohedral axes a b c a b
g ? 90o Has a 3-fold axis.
Hexagonal a b ? c a b 90o g 120o
has a 6-fold axis.
Trigonal Hexagonal axes a b ? c a b 90o
g 120o but has only 3-fold axis.
19
- Non-primative unit cell.
- Four clases
- - Primitive unit cell (P).
- - Plane centred unit cell (A, B or C).
- - Body centred unit cell (I).
- - Face centred unit cell (F).
- In combination with previous 7 primitive cells
leads to 14 Bravais lattices.
P
A
I
F
20
Why bother with non-primitive cells?
- The blue unit-cell is a primitive unit cells
with ½ the volume of the A unit cell. - - However some symmetry of the total unit cell is
lost in making the choice of the blue cell. - - a ? b ? g in this choice but a b g 90o in
the other.
c
b
a
21
- Number of ways of packing a crystal.
- 14 Bravais lattices.
- Additional crystallographic symmetries
- - Rotation, Inversion, mirror planes.
- Form the set of 32 point-groups.
- - Addition of glide screw axes.
- Leads to 230 space-groups!
22
- Equivalent positions
- Suppose (x, y, z) is a normal fractional
coordinate. - For each symmetry operation within the
space-group there exists another position (x,
y, z) within the unit cell which is rellated to
(x, y, z) by symmetry. - (x, y, z) is called an equivalent position.
- eg. 2-fold rotation about a takes (x, y, z) out
of the unit cell. - A translation by b c returns it into the unit
cell. - This is (x, y, z) is an equivalent position
to (x, y, z)
23
- The assymeteric unit.
- The smallest non-repeating unit within a crystal
is called the assymeteric unit. - A unit-cell may have several copies of an
asymmeteric unit, all of which are related by
(non-translational) crystallographic symmetry. - Each atom within the assymeteric unit is related
by a symmetry operation (ie. equivalent position)
to an identical atom of another assymeteric unit.
- The number of assymeteric units is determined by
the number of equivalent positions within the
space group!
24
- Special positions.
- A special position is a place where a point is
taken to itself under a symmetry operation. - ie. (x, y, z) ? (x,y,z) (x, y, z).
- - Electron density at a special position can be
problematic to interpret. - - Direct methods for phasing dont work when the
atoms lie on special positions.
- eg. 2-fold rotation about a leaves (0, y, z) on
the a-axis. - Thus (0, y, z) is a speical position.
25
- Non-crystallographic symmetry.
- In addition to the crystallographic symmetry
molecules may repeat with non-crystallographic
symmetry. - eg. A virus capsid has 5-fold symmetry, which is
not a crystallographic symmetry. - Two molecules may pack against each other in a
slightly skewed manner fail to give a perfect
crystallographic symmetry.
26
- Notation.
- Space groups are first labelled according to
lattice ie. P, A (B or C), F or I. - Next labelled relative to the highest symmetry
axes. - - P6 means a primitive lattice with one 6-fold
axis. - - P222 means a primitive lattice with three
two-fold axes. - - F means a face-centred lattice with no
additional symmetry. - A subscript then is used to denote screw axes.
- - P63 means a six-fold screw axis.
- - P212121 means three two-fold screw axes.
27
- Packing of protein crystals.
- The assymeteric unit may contain
- One molecule.
- Several molecules with different conformations.
- Identical domains of one molecule.
- Amino acids are chiral.
- Inversion mirror symmetry operations are not
allowed. - Only 65 of the possible 230 space groups are
available. - Protein crystals are loosely packed.
- Approximately 50 to 80 of a protein crystal
is solvent. - Crystal conformations well approximate
physiological conformations.
28
- Summary
- To fully describe a crystal packing you need
- Length of unit cell axes, a, b c.
- Unit cell angles, a, b g.
- Crystal packing Primitive, plane centred, body
centred or face centred? - Additonal symmetry rotation, screw axes,
mirror, inversion glide symmetries. - Must identify the assymeteric unit.
- Must identify any non-crystallographic symmetry.
- There are 14 possible crystal lattices.
- 230 possible space groups.
- Macromolecules can occupy only 65 space groups
due to their chirality.