OKAY - PowerPoint PPT Presentation
OKAY
If kchem krev or krev kchem, then will not have a current. for ... What if redox species (O) is adsorbed on electrode surface? Q. Qads. QDL (blank) only S.E. ... – PowerPoint PPT presentation
Title: OKAY
1
10-1
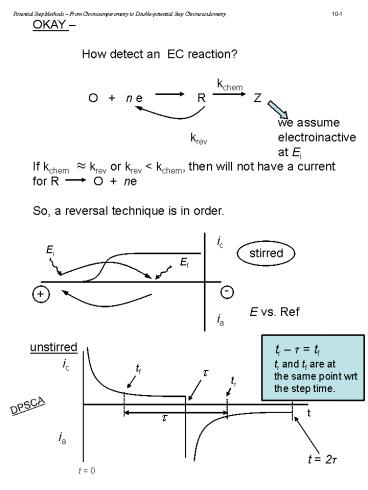
Potential Step Methods From Chronoamperometry
to Double-potential Step Chronocoulometry
OKAY How detect an EC reaction?
kchem O n e R
Z we assume
krev electroinactive at Ei If kchem
krev or krev lt kchem, then will not have a
current for R O ne So, a reversal
technique is in order.
ic
Ei
stirred E vs. Ref
Ef
-
ia
unstirred
tr t tf
ic
tr and tf are at the same point wrt the step
time.
tf
tr
DPSCA
t
ia
t 2t
t 0
2
10-2
Potential Step Methods From Chronoamperometry
to Double-potential Step Chronocoulometry
recalling
we see
The maximum ratio, if R is stable. If
, R unstable at this value.
decreasing stability of R 1. larger kchem
2. larger
1.0
0.293
more stable
1
2
So, a chemically irreversible reaction can be
made reversible and DC if t is small enough.
But, if t too small, then tf is small enough
such that reaction looks ET limited.
3
10-3
Potential Step Methods From Chronoamperometry
to Double-potential Step Chronocoulometry
Measuring instantaneous currents is not always
easy.
Chronocoulometry (CC)
charge
Ef E Ei
i
Q
t
0 t
t
For O n e R, plot Q
vs. t1/2 If plot linear, then
reaction is D-Controlled
Q
QDL
QDL
t1/2
4
10-4
Potential Step Methods From Chronoamperometry
to Double-potential Step Chronocoulometry
What if redox species (O) is adsorbed on
electrode surface?
Q
Qads
QDL (blank) only S.E.
t1/2
is 2 D conc. in mol/cm2
We assume that Not so,
due to inherent Capacitance of adsorbed species
on surface.
How deal with this situation?
Double Potential Step Chronocoulometry
Q
t
5
10-5
Potential Step Methods From Chronoamperometry
to Double-potential Step Chronocoulometry
Qf vs. t
For adsorption
Qads QDL
sec1/2
QDL iff R not adsorbed!
Now get Qads by subtraction.
What about stability of R? (What else is DPSCC
good for?)
As kchem becomes faster
1.0
Completely irreversible
0 0
1.0
2.0
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.













![READ[PDF] It's Okay If You Don't Like Softball It's Kind Of A Smart P PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10068921.th0.jpg?_=202407010210)
![READ[PDF] It's Okay If You Don't Like Softball It's Kind Of A Smart P PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10067547.th0.jpg?_=20240628035)
![READ[PDF] It's Okay If You Don't Like Softball It's Kind Of A Smart P PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10060687.th0.jpg?_=20240621099)
![READ[PDF] It's Okay If You Don't Like Softball It's Kind Of A Smart P PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10060413.th0.jpg?_=20240621056)









![[PDF] DOWNLOAD It's Okay If You Don't Like Golf It's Kind Of A Smart Peo PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10068911.th0.jpg?_=20240701028)
![[PDF] DOWNLOAD It's Okay If You Don't Like Golf It's Kind Of A Smart Peo PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10067546.th0.jpg?_=202406280110)

![[PDF] DOWNLOAD It's Okay If You Don't Like Golf It's Kind Of A Smart Peo PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10060412.th0.jpg?_=20240621055)
![[PDF] DOWNLOAD It's Okay If You Don't Like Golf It's Kind Of A Smart Peo PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10060686.th0.jpg?_=20240621098)