Pump-probe spectroscopy: fast versus slow nuclear dynamics - PowerPoint PPT Presentation
1 / 39
Title:
Pump-probe spectroscopy: fast versus slow nuclear dynamics
Description:
Pump-probe spectroscopy: fast versus slow nuclear dynamics 2D Born Oppenheimer approximation: H-O stretch motion (fast subsystem): 1D Nuclear Hamiltonian of ... – PowerPoint PPT presentation
Number of Views:387
Avg rating:3.0/5.0
Title: Pump-probe spectroscopy: fast versus slow nuclear dynamics
1
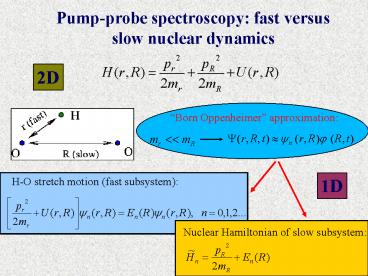
Pump-probe spectroscopy fast versus slow nuclear
dynamics
2D
1D
2
Pump-probe spectroscopy in the framework of BO
Pump field
Water Dimer
mixes two lowest OH vibrational states
Dynamics of femtosecond O-O stretch motion
3
(No Transcript)
4
(No Transcript)
5
(No Transcript)
6
Property Toolbox
magnetic
internal
external
electric
linear
time-dep
nonlinear
Time-indep
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
Dependence of collisional dephasing rate on
photon detuning
Homogeneous broadening
Life-time broadening
Collisional dephasing rate
Kenji Kamada measurements
14
Example PRL-101
Ab initio results
AM1 geometry/6-31G/DFT Quadratic Response
1280 GM at G 0.1 eV
15
Non-linear pulse propagation
T (1 W/cm2) 0.994
L 5 mm
t 140 fsec
G 0.1 eV
16
Non-linear pulse propagation
17
Non-linear pulse propagation
Exponential decay of red wing of linear
absorption profile
In case of Lorentzian decay TPA cross section is
unrealistically high
Inhomogeneous broadening of TPA spectra is not
considered
18
Sensor ProtectionProtection against lasers
19
The Project Group/Co-Workers
Preparation of materials
Modeling
- Dr. Bertil Eliasson, UmU, Sweden
- Marcus Carlsson, PhD student
- Dr. Eva Malmström, KTH, Sweden
- Robert Vestberg, PhD student
- Robert Westlund, PhD student
- Dr. Stephane Parola, UCBL, France
- Marcus Örtenblad, PhD student
- Prof. Hans Ågren, KTH, Sweden
- Oscar Rubio Pons, PhD student
- Peter Cronstrand, PhD student
- Dr. Patrick Norman, LiU, Sweden
- Johan Henriksson, PhD student
Characterization
- Prof. Mikael Lindgren, NTNU, Norway
- Dr Jonas Örtegren, Post Doc
- Eirik Glimsdal, Dipl. Stud
- Dr. Anders Eriksson, FOI, Sweden
- Dr. Cesar Lopes, FOI, Sweden
Optical Equipment design
- Dr. Henrik Ludwigs, Saab Tech AB
20
Project Goals
Design and preparation of solid-state materials,
with ability to clamp the transmitted energy 1
?J _at_ 60 photopic transmission, for protection of
eyes, E/O sensors and NVG against µs ps pulses.
- Preparation
- Dendrimers
- Nanohybrid materials
- Solid-state glass materials
- Characterization
- Transmission
- OPL - Clamping
- Mechanisms
- Modeling
- The matrix - influence
- Concentration
- New nanomaterials
21
Solid-state optical limiting materials -Hybrid
nanocomposites-
- Enhanced chemical, physical and mechanical long
term stability - Enhanced performance
- Environmentally friendly composition
- Shape processability
Synthesis Precursor Dendrimer ligand
Synthesis Precursor Me-organic compound
Synthesis Precursor Nanohybrid material
Preparation Glass material
Solid-state material Hybrid material Organic
matrix
Solid-state material Hybrid material Inorganic
matrix
22
Preparation of solid materials
- Dendrimers
- Coating
- Preparation of solids, organic matrix
- Glass materials
- Nanohybrid precursors
- Class I and II materials
23
Class II nanohybrid materials
Si(OR)4 H2O
Class II solid-state material
24
Optical characterization
- OPL characterization (standard f/5 set-up)
- Spectroscopy
- Optical absorption (UV-VIS and excited state
absorption) - Steady state and time-resolved luminescense
spectroscopy
25
Sample preparation
Precision saw machine (Isomet 1000) and polishing
machine (Logitech PM2)
26
Results year 1
Pt-Thiacalixarenes 50 mM och 12.5 mM
27
Results year 1
Synthesis and characterization of new NLO
chromophores Dendrimer capped Pt-aryl-ethynyls
preliminary OPL
28
Results year 1
Preparation of solid OPL materials sol-gel
PtG2
Boltorn H30
29
Scientific output2003 - 2004
- 25 publications
- P. Norman and H. Ågren First principles
quantum modeling of optical power limiting J.
Comp. Theoretical Nanoscience, 2004 (in press) - R. Vestberg, A. Nyström, M. Lindgren, E.
Malmström and A. Hult Encapsulation of
porphyrin cores by bis-MPA dendrons Chemistry
of Materials 16, (2004), 2794 - P. Cronstrand, P. Norman, Y. Luo and H.
Ågren Few states models for three-photon
absorption J. Chem. Phys. 121, (2004), 2020 - R. Vestberg, C. Nilsson, C. Lopes, B. Eliasson
and E. Malmström Thiophene cored bis-MPA
dendrimers for OPL applications Journal of
Polymer Science Part A Polymer Chemistry (2004) - R. Vestberg, A. Eriksson, C. Lopes, M. Lindgren
and E. Malmström Novel dendrimer-capped
Pt-acetylides for OPL SPIE 5621, 2004
30
Porphyrin-cored 2,2-bis(methyole)propionic
acid dendrimers
2,2-bis(methylol)propionic acid (bis-MPA)
dendrimers have been obtained by the direct
addition of bis-MPA dendrons to free-base and
Zn-porphyrins. The growth of dendrimers in the
case of Zn-TPP tetrakis(4-hydroxyphenyl)-porphin
e is shown here.
31
Fluorescence of dendrimers in THF
No difference in emission for different
generations of free base. For Zn-cored
porphyrins the shoulder at 650 nm increases with
increasing generation.
Free-base TPP in G3
Zn-TPP in Gx dendrimers
32
We have compared dendrimer spectra with FBP and
ZnP emission spectra in different solvents and
solid matrices and also with IR and Raman spectra
(nonresonance and normalRaman). Comparative
theoretical study of all these spectra, including
simple models of dendrimers (Zn-TPP) at different
levels (DFT and AM1)permits us the following
explanations
33
This vibration is observed in Raman spectra at
1609 cm-1 and is identified with 1614 cm-1
vibronic 0-1 band in fluorescence (n10 of ag
type). It is seen as a shoulder at 720 nm for
free-base-TPP fluorescence in G3 dendrimer. It is
shifted in TPP to lower frequency. The band is
induced by large FC factor. No Herzberg-Teller
contribution (ag)
34
In Zn-TPP molecule this mode is mixed with the
phenyl stretchings. Phenyl rings are
out-of-porphpyrin-plane. When they bear bulky
dendric MPA-substitutients this strongly
influences electronic cloud of the Zn-porphpyrin
chromophore The Herzberg-Teller mechanism now
contributes more to intensity of vibronic
line. It influence mixing of the S1(Qx) and the
Soret states.
Vibronic shoulder at 660 nm in ZnTPP
fluorescence its intensity increases with
dendrimer generation. It is induced by
Herzberg-Teller effect In Zn-P molecule this
band is changed in comparison with FBP, since it
includes now Zn-N vibrations (asymmetric wagging
movement). This is b2g mode which includes also
Ca-Cm vibrations in methyne bridges.
35
Among other low-frequency vibronic bands there is
the nu27 755cm-1, which also includes the
vibrations in methyne bridges and Zn
movement. The similar Herzberg-Teller mechanism
contributes to intensity of this vibronic line
with growing dendric MPA-substitutients. It gives
additional emission band (two-hump shoulder) in
G5 fluorescence
36
This is ullustrated by Zn-TPP vibrations
calculated at AM1 level
37
Phosphorescence of free-base porphin and
Zn-porphyrin. The efficient inter-system
crossing of porphyrins, which maintain a high
concentration of triplet-excited molecules is
used now in a wide variety of applications from
photodynamic therapy to nonlinear optical
devices. We have explained for the first time
the low phosphorescence efficiency of porphyrins
without heavy ions by DT DFT calculations. We
have obtained a slow radiative rate constant of
the lowest triplet state, 3B2u, of free-base
porphin phosphorescence (about 10-3 s-1), which
is in agreement with experimental
estimations. Phosphorescence of free-base porphin
is determined by emission from the most active Tz
spin sublevel, where z-axis coinsides with the
N-H...H-N bond direction. It is polarised
perpendicular to the molecular plane. Such a slow
radiative decay is very unusual for a molecule
wich possesses lone pairs of electrons at
nitrogen atoms and a number of excited np states
in the near UV region. It is explained by
destructive interference of S-S and T-T
contribution.
38
(No Transcript)
39
(No Transcript)