Cell EMF PowerPoint PPT Presentation
Title: Cell EMF
1
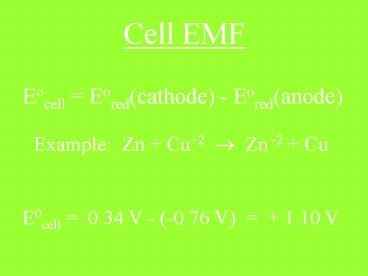
Cell EMF
Eocell Eored(cathode) - Eored(anode)
Example Zn Cu2 ? Zn2 Cu E0cell 0.34
V - (-0.76 V) 1.10 V
2
A voltaic cell is based on two half-reactions
Cd2/Cd Sn2/Sn Which half-reaction takes
place at the cathode? Which half-reaction takes
place at the anode? What is the standard cell
potential?
3
A voltaic cell is based on two half-reactions
Cd2/Cd Sn2/Sn Which half-reaction takes
place at the cathode? Which half-reaction takes
place at the anode? What is the standard cell
potential?
Sn2 2e- ? Sn
Cd ? Cd2 2e-
0.267 V
4
(No Transcript)
5
Spontaneity of Redox Reactions
Eo Eored(reduction) - Eored(oxidation)
E0 () spontaneous E0 (-) nonspontaneous
6
Cu 2H ? Cu2 H2
Calculate the value of E0.
7
Cu 2H ? Cu2 H2
Calculate the value of E0.
E0 -0.34 V NOT SPONTANEOUS
8
EMF and Free-Energy Change
?G -nFE
n a positive (the of electrons
transferred) F Faradays constant. 1F
96,500 J/V-mol E EMF
9
Use the standard reduction potentials to
calculate the standard free-energy change, ??Go,
for the following reaction
4Ag O2 4H ? 4Ag 2H2O
10
Use the standard reduction potentials to
calculate the standard free-energy change, ??Go,
for the following reaction
4Ag O2 4H ? 4Ag 2H2O
?Go -(4)(96,500J/V-mol)(0.43V) - 170 kJ/mol
11
The Nernst Equation
(at 298 K)
12
Calculate the emf at 298K generated by the
following cell, when (Cr2O7-2) 2.0 M, (H)
1.0 M, (I-) 1.0 M, and (Cr3) 1.0 x 10-5 M
Cr2O7-2(aq) 14H(aq) 6I-(aq) ? 2Cr3(aq)
3I2(s) 7H20(l)
13
Calculate the emf at 298K generated by the
following cell, when (Cr2O7-2) 2.0 M, (H)
1.0 M, (I-) 1.0 M, and (Cr3) 1.0 x 10-5 M
Cr2O7-2 14H 6I- ? 2Cr3 3I2 7H20
E 0.79 V - 0.0592V/6 log(5.0 x 10-11) E
0.89 V
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.