The Periodic Table - PowerPoint PPT Presentation
1 / 20
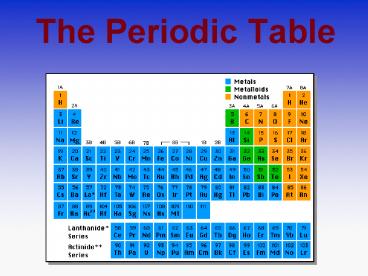
Title: The Periodic Table
1
- The Periodic Table
2
The Language of Chemistry
- CHEMICAL ELEMENTS
- pure substances that cannot be changed into
other substances.
Aluminum
Bromine
Sodium
3
The Language of Chemistry
- The elements, their names, and symbols are given
on the PERIODIC TABLE - How many elements are there?
4
The Periodic Table
- Dmitri Mendeleev (1834 - 1907)
5
Dmitri Mendeleev (1869)
- In 1869 Mendeleev and Lothar Meyer (Germany)
published nearly identical classification
schemes for elements known to date. - Mendeleev gets most credit as he could make
accurate predictions about elements which he
thought must exist, but were unknown. - The Periodic Table is based on the similarity of
properties and reactivities exhibited by certain
elements. - Later, Henri Moseley (England, 1887-1915)
established that each elements has a unique
atomic number, which is how the current periodic
table is organized.
6
Glenn Seaborg(1912-1999 )
- Discovered 8 new elements.
- Only living person for whom an element was named.
7
Periods in the Periodic Table
The rows are the Periods of the Periodic
Table. As you go down the Periodic Table the
elements become more reactive.
8
Groups in the Periodic Table
The elements in the columns are the Groups of the
Periodic Table.
Elements in groups react in similar ways!
9
Group 1 Alkali Metals
Reaction of potassium H2O
Cutting sodium metal
10
Group 2 Alkaline Earth Metals
Magnesium
11
Group 7 The Halogens Fluorine, Chlorine,
Bromine, Iodine
12
Group 8 The Noble GasesHelium, Neon, Argon,
Krypton, Xenon, Radon
- Lighter than air balloons
- Neon signs
- Very Unreactive
13
Transition Elements
- Lanthanides and actinides
Iron in air gives iron(III) oxide
14
Names of Elements
- Each element is given a separate name
- Hydrogen, carbon, oxygen, iron and aluminium are
some well known elements - Several elements, such as curium and einsteinium
are named after well known scientists - Others such as francium, germanium and americium
are named after their country of discovery
15
Where do all the Chemical Elements come from?
- Only the first 92 elements occur naturally and
can be found in the Earth, air or water. - Nuclear scientists have produced these chemical
elements inside nuclear reactors - Many of these new elements do not last very long
and often break down by giving off nuclear
radiation.
16
Atomic Symbols
- Each element is also given a one letter or two
letter symbol which is used as a shorthand way of
writing the element - Where a one letter symbol is used, it is written
in upper case - Where two letters are used, the first is written
in upper case, and the second in lower case
17
Atomic Symbols continued
- Some element symbols are clearly abbreviations of
the elements names e.g. carbon C, oxygen O,
calcium Ca - Others, such as iron Fe, sodium Na, mercury
Hg, are abbreviations of their Latin names
ferrum, natrium and hydrargyrum
18
Atomic Number
- Each element has a different ATOMIC NUMBER this
is identified at the top of their square
13
Al
26.981
19
Abundance of the Elements
- ABUNDANCE means how much there is of it.
- There are differences between the abundance of
elements in the Universe, the Earth and in the
human body.
All other elements 3
Hydrogen 60
Helium 37
20
All other elements mainly metals 12
Oxygen 47
Iron 5
Aluminium 8
Silicon 28
Calcium 2
All other elements mainly metals 2
Nitrogen 2
Hydrogen 10
Oxygen 65
Carbon 18