morphine - PowerPoint PPT Presentation
Title:
morphine
Description:
Title: PowerPoint Presentation Author: Loyd Bastin Last modified by: Loyd Bastin Created Date: 9/3/2005 6:03:04 PM Document presentation format: On-screen Show (4:3) – PowerPoint PPT presentation
Number of Views:159
Avg rating:3.0/5.0
Title: morphine
1
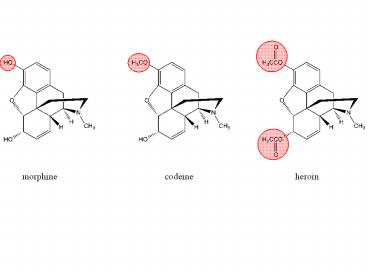
morphine
codeine
heroin
2
Ibuprofen
R form non-active side effects
S form active anti-inflammatory
3
R.B. Woodward (1917-1979)
- 1st modern synthetic organic chemist
- Probably greatest organic chemist
- 1965 Nobel Prize in Chemistry
- outstanding achievements in the art of organic
synthesis - Also made VERY important observations in the
development of the Woodward-Hoffman rules of ring
closure - 1st step in the application of quantum mechanics
to organic molecules - 1981 Nobel Prize in Chemistry (Roald Hoffmann)
4
R.B. Woodward (Early Career)
5
R.B. Woodward (Later Career)
6
R.B. Woodward
7
K.C. Nicolaou
- Penn (1977-1989)
- Scripps Research Institute and UC-San Diego
(1989-present) - Modern day R.B. Woodward
8
K.C. Nicolaou Taxol
- Isolated in 1967 from bark of Pacific yew tree
- Lung, ovarian, breast, head and neck cancer
11 stereocenters gt 211 2048 stereoisomers 2
rings 1 bicyclic ring
9
K.C. Nicolaou Brevotoxin B
- Neurotoxin that binds to voltage-gated sodium
channels in nerve cells - Naturally found in Karenia brevis which are
marine organisms typically found in fish
23 stereocenters gt 223 8,400,000
stereoisomers 11 trans-fused rings 83 steps, 12
years 91 yield for each step but 0.043 total
yield
10
K.C. Nicolaou Maitotoxin
- Neurotoxin that binds to calcium channels
- Naturally produced by Gambierdiscus toxicus which
are marine organisms typically found in fish
94 stereocenters gt 294 1.98 x 1028
stereoisomers 31 trans-fused rings
11
Atomic Orbitals
n l ml Orbital Name
1 0 0 1s
2 0 0 2s
2 1 -1 2p
2 1 0 2p
2 1 1 2p
3 0 0 3s
3 1 -1 3p
3 1 0 3p
3 1 1 3p
3 2 -2 3d
3 2 -1 3d
3 2 0 3d
3 2 1 3d
3 2 2 3d
n l Orbital Name
1 0 1s
2 0 2s
2 1 2p
3 0 3s
3 1 3p
3 2 3d
12
(No Transcript)
13
(No Transcript)
14
Linus Pauling (1901-1994)
- Probably greatest chemist of the 20th century
- Only person too win 2 unshared Nobel Prizes and
only person to win 2 unrelated Nobel Prizes - 1954 Chemistry
- 1962 - Peace
- The Nature of the Chemical Bond
- Protein Structure
- Antibody Structure and Process
- Molecular cause of Sickle-cell anemia
- Electronegativity
- Resonance
- Vitamin C and the Common Cold
15
The Periodic Table and Electronegativity
16
Common Bonding Situations
Hydrogen
1 bond
Carbon
4 bonds (neutral and 8 electrons)
Reactive Carbon Species
17
Nitrogen
3 bonds and one unshared pair of electrons
Other relatively stable species
18
Oxygen
2 bonds and 2 unshared electron pairs
Other relatively stable species
hydronium ion
19
Halogens
1 bond and 3 unshared electron pairs
also 4 unshared pairs and negative charge
20
Table 2-6a, p. 51
21
Table 2-6b, p. 51
22
(No Transcript)