Functional Groups PowerPoint PPT Presentation
1 / 18
Title: Functional Groups
1
Functional Groups
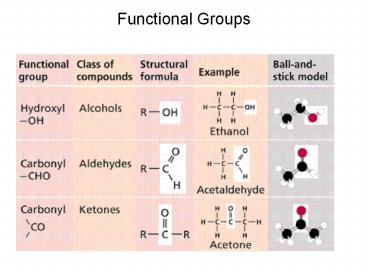
2
(No Transcript)
3
aldehyde
- Definition an organic compound containing a
Carbonyl group ( CO ).
The aldehyde group is generaly written -CHO
- the name Aldehyde comes from Alcohol-dehydrogenate
d meaning the loss of hydrogen -
Benzaldehyde aroma in cherries, almonds, perfumes
4
Carboxyl Group
- Definition a Carboxyl group ( -COOH), double
bonded to an oxygen and single-bonded to the
oxygen of the hydroxyl group - Polar, water-soluble
- The H dissociates and thus has acidic
properties. Compounds with this functional group
are called carboxylic acids
The Carboxyl group is generally written -COOH
Carboxylic acids are the most important acids of
organic chemistry. They are present or in derived
form in many natural substances Amino acids
(building blocks of proteins). Note that the
amino acids also contain an amine group. Below
is given the structure of alanine Fatty acids
(building blocks of lipids) are long aliphatic
chains terminated by the carboxyl group.The
structure of stearine is given below.
Fatty acid
Glutamic acid
5
Carbonyl Group
- Definition functional group consisting of a
carbon atom double-bonded to oxygen - (-CO)
- Polar, involved in H-bonding and molecules with
this functional group are water-soluble - Found in sugars
- If the carbonyl is at the end off the carbon
skeleton-the compound is an aldehyde - If the carbonyl is at the end of the carbon
skeleton, the compound is a ketone
6
Alcohol
- Definition an alcohol is an organic compound
containing a hydroxyl group ( O-H ).The molecule
on the left (methanol) shows the alcohol
functional group
OH-CH2-CH2-OH ethylene glycol (antifreeze)
7
Amine
Definition an Amine is a derivative of ammonia (
NH3 ) in which hydrogen atoms are replaced by R
group
caffeine
Alanine cysteine
8
Phosphate group
- Definition functional group which is the
dissociated form of phosphoric acid - Has acid properties since it loses H (protons)
- Polar and soluble in water
- Important in cellular energy storage and transfer
(ATP)
9
Sulfhydryl group
- Definition functional group consisting of an
atom of sulfur bonded to an atom of hydrogen - Help stabilize structure of proteins-disulfide
bridges in the tertiary structure of proteins - Called thiols when found in organic compounds
10
Carbon atoms and molecules of carbon
hexane
Methane ethane
Branching-isohexane
Ring-cyclohexane
Single, double, triple bonds
11
Isomers have the same molecular formula but
different structures and different properties
- STRUCTURAL
BOTH HAVE THE SAME MOLECULAR FORMULA C6H14 BUT
DIFFERENT STRUCTURAL FORMULAS
12
Structural isomers
13
- GEOMETRIC
These two chlorine atoms are locked on opposite
sides of the double bond. This is known as the
trans isomer the two chlorine atoms are locked on
the same side of the double bond. This is know as
the cis isomer. (Hint if you build models and
have to take it apart-geometric isomer)
free rotation about single bonds these two
structures represent the same molecule (Hint if
you build model-you just have to rotate c-c bond-
not an isomer)
14
Cis/trans geometric isomers
- In triglycerides fatty acids may contain double
bonds, which can be in either the cis or trans
configuration . - Fats with at least one double bond between carbon
atoms are unsaturated fats. When some of these
bonds are in the cis configuration, the molecules
cannot pack tightly, so they remain liquid (oil)
at room temperature. - triglycerides with trans double bonds (called
trans fats), have are linear fatty acids that are
able to pack tightly together at room temperature
and form solid fats.
15
- ENANTIOMERS
- Whenever a carbon atom has four different
structures bonded to it, two different molecules
can be formed. (chiral means the central C is
bonded to 4 different groups or atoms) - EXAMPLE the amino acid alanine. Bonded to its
alpha carbon atom are - a carboxyl group (COO-)
- an amino group (NH3)
- a methyl group (CH3)(its R group)
- a hydrogen atom
- If you orient the molecule so that you look along
it from the COO- group to the NH3 group, the
methyl (R) group can extend out - to the left, forming L-alanine (shown below on
the left) or - to the right, forming D-alanine (on the right).
16
- Although they share the same chemical formula,
they are not interchangeable any more than a
left-hand glove is interchangeable with
right-hand glove. - 19 of the 20 amino acids used to synthesize
proteins can exist as L- or D- enantiomorphs. The
exception is glycine, which has two
(indistinguishable) hydrogen atoms attached to
its alpha carbon. - L amino acids are used exclusively for protein
synthesis by all life on our planet. (Some D
amino acids are used for other purposes.)
17
- The function of a protein is determined by its
shape. - A protein with a D amino acid instead of L will
have its R group sticking out in the wrong
direction. - Many other kinds of organic molecules exist as
enantiomers. Usually only one form is active in
biological systems. For example, if one form
binds to a receptor protein on the surface of a
cell, the other probably cannot. - Cells usually synthesize only one form. However,
chemical synthesis in the laboratory or
pharmaceutical factory usually produces equal
amounts of the two enantiomers called a racemic
mixture. - Example The drug albuterol (e.g., Proventil)
contains equal amounts of two enantiomers. Only
one of them is effective, and the other may be
responsible for the occasional unpleasant
side-effects associated with the drug (which is
used to dilate the bronchi, e.g, during an attack
of asthma).
18
Chiral compounds
- Some chemical compounds have optical activity in
the sense that these compounds have the ability
to rotate the plane of polarized light. Polarized
light has light waves all traveling parallel to
each other. Ordinary light has light waves
traveling in all directions. When polarized light
is passed through a solution of an optically
active compound, the plane of polarization is
rotated to the right or the left. The angle of
rotation can be measured in a polarimeter. - An optically active organic compound can be
identified by finding a chiral carbon. A chiral
carbon is one that has four different "groups"
attached to it. The groups can be anything from a
single H to functional groups to one or more
other carbons. See bromochloroiodomethane on the
left - it has 3 halogens and one hydrogen. - In relatively complicated compounds, each carbon
must be examined carefully to determine whether
it is chiral. Some compounds may have two or more
chiral carbon centers such as in carbohydrates. - See glyceraldehyde Carbon 1 has only three
groups attached. Carbon 3 has two hydrogens
which count as 2 of the same groups. Finally,
carbon 2 has four different groups attached.