Chap. 7. Problem 2. - PowerPoint PPT Presentation
Title:
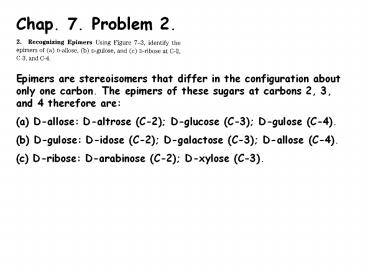
Chap. 7. Problem 2.
Description:
D-allose: D-altrose (C-2); D-glucose (C-3); D-gulose (C-4). D-gulose: D-idose (C-2); D-galactose (C-3); D-allose (C-4). D-ribose: D-arabinose (C-2); D-xylose (C-3). – PowerPoint PPT presentation
Number of Views:66
Avg rating:3.0/5.0
Title: Chap. 7. Problem 2.
1
Chap. 7. Problem 2.
- Epimers are stereoisomers that differ in the
configuration about only one carbon. The epimers
of these sugars at carbons 2, 3, and 4 therefore
are - D-allose D-altrose (C-2) D-glucose (C-3)
D-gulose (C-4). - D-gulose D-idose (C-2) D-galactose (C-3)
D-allose (C-4). - D-ribose D-arabinose (C-2) D-xylose (C-3).
2
Chap. 7. Problem 6.
- Cellulose and glycogen Both of these compounds
are homopolysaccharides of D-glucose. Cellulose
is a linear polymer, whereas glycogen is a
branched polymer. O-glycosidic linkages in
cellulose are exclusively (ß1?4). O-glycosidic
linkages in glycogen are (?1?4) in the main
chains and (?1?6) at branch points. - D-glucose and D-fructose Both of these
monosaccharides are hexoses. D-fructose is a
ketose, and D-glucose is an aldose. - Maltose and sucrose Both of these sugars are
disaccharides. Maltose contains two (?1?4) linked
D-glucose units. Sucrose contains (?1? 2ß) linked
D-glucose and D-fructose units. Maltose is a
reducing sugar sucrose is not.
3
Chap. 7. Problem 9.
Straight-chain fructose can cyclize to either the
pyranose or furanose forms. The observations in
the problem can be explained if heating converts
more of the fructose to its furanose form, which
is less sweet than the pyranose form.
4
Chap. 7. Problem 10.
Although glucose oxidase is specific for the ß
anomer of D-glucopyranose, the enzyme can
ultimately oxidize all of the glucose in solution
because the ß and ? anomers are in equilibrium
via mutarotation. Glucose oxidase is more
accurate than Fehlings reagent for measuring
glucose in the blood, because the enzyme is
specific for glucose and does not detect other
reducing sugars (e.g., galactose) that react with
Fehlings reagent.
5
Chap. 7. Problem 13.
Lactose (Gal(ß1?4)Glc) exists in two anomeric
forms because the free anomeric carbon (C-1) in
the glucose residue can undergo mutarotation. In
sucrose (Glc(?1? 2ß)Fru), the anomeric carbons of
both monosaccharides are linked via an
O-glycosidic bond. Thus, sucrose lacks a free
anomeric carbon that can intercovert between ?
and ß forms via mutarotation.
6
Chap. 7. Problem 15.
N-acetyl-ß-D-glucosamine is a reducing sugar
because it contains a free anomeric carbon at C-1
that can open to the straight-chain form and
therefore can be oxidized. D-gluconate is not a
reducing sugar because its anomeric carbon at C-1
is already oxidized to the level of a carboxylic
acid. The disaccharide GlcN(?1? 1?)Glc is not a
reducing sugar because it lacks a free anomeric
carbon. The anomeric carbons of both glucose
units in this compound are tied up in an
O-glycosidic linkage and cannot open to the
straight-chain forms required for oxidation.
7
Chap. 7. Problem 17.
In glycogen, the (?1?4) linkages in the main
chains produce bends in the chains and limit the
formation of long fibers. Branching also favors
the formation of a globular, granular structure.
Many of the hydroxyl groups of glucose units in
the polymer are exposed to
water and are hydrated, which explains why
glycogen can be dispersed in hot water to make a
turbid solution. In cellulose, glucose units are
linked via (ß1?4) linkages. This allows the
polymer to adopt an extended conformation in
which parallel chains are held together via
numerous interchain hydrogen bonds. Water is
mostly excluded from cellulose which forms
insoluble, tough fibers. Cellulose therefore is
well suited to take on a structural, supportive
role in plants. Glycogen, due to branching and
hydration, is well suited to serve as an energy
repository from which glucose units can readily
be liberated by enzymatic cleavage.
8
Chap. 7. Problem 22.
Chondroitan sulfate contains a large number of
negatively charged carboxylate and sulfate
functional groups. In solution, these negative
charges repel one another and force the molecule
into an extended conformation. Chondroitan
sulfate also is extensively hydrated due to the
prevalence of polar and charged groups, and this
increases the volume occupied by this molecule in
solution. The dehydrated solid form of
chondroitan sulfate is produced by removal of
water molecules and addition of positively
charged counterions such as sodium ion which
masks the negative charges of the polymer. In
this form, the volume of the molecule is greatly
reduced from that observed in solution.
9
Chap. 7. Problem 26.
Oligosaccharides composed of five different
monosaccharide residues actually can produce a
greater variety of structures than oligopeptides
composed of five different amino acid residues.
Oligopeptides are unbranched polymers in which
every amino acid is linked via a simple peptide
bond. In oligosaccharides, O-glycosidic linkages
can be formed using several different hydroxyl
groups in the monomer units, and each glycosidic
bond can be either ? or ß. In addition, branched
structures are possible. Overall, monosaccharide
units in oligosaccharides can be combined in more
ways than the amino acids of an oligopeptide.