Reactant PowerPoint PPT Presentations
All Time
Recommended
reactant + reactant product + product Test 1 water hydrochloric acid carbon dioxide calcium carbonate calcium chloride Test 2 hydrogen hydrochloric acid
| PowerPoint PPT presentation | free to download
Limiting Reactant Stoichiometry Refresher Recall: The mole ratio can be used to determine the quantity (moles) or mass of reactants consumed and products formed ...
| PowerPoint PPT presentation | free to download
Limiting Reactant Theoretical and Percent Yield Limiting Reactant: Cookies 1 cup butter 1/2 cup white sugar 1 cup packed brown sugar 1 teaspoon vanilla extract 2 eggs ...
| PowerPoint PPT presentation | free to view
Limiting Reactants & Percent Yield. Continuation from Stoichiometry. A. Types of Reactants ... B. Reactants Stoichiometry Problems. 4 NH3 5 O2 4 NO 6 H2O ...
| PowerPoint PPT presentation | free to view
Divide the moles of each reactant by its stoichiometric coefficient and then compare them ... Step 2. Divide moles by its stoichiometric coefficient ...
| PowerPoint PPT presentation | free to view
Limiting Reactants and ICE Charts Chemistry Cake You have 20 cups of flour, 8 cups of sugar, 30 litres of milk and 48 eggs in your kitchen. The recipe for chemistry ...
| PowerPoint PPT presentation | free to download
Nitrogen monoxide can be prepared by the oxidation of ... Glucosamine sulfate. Hydrochloride. Citrate. Sulfate. Tartrate. Oxycodone HCl. sildenafil citrate ...
| PowerPoint PPT presentation | free to view
These calculations are used in both, General and Organic Chemistry ... Definitions. Three methods used to determine the limiting reactant ...
| PowerPoint PPT presentation | free to view
Which ingredient ran out first and limited the number of cakes you could make? ... 15.0 mole Ga and 12.0 mole O2 react. Find. the limiting reactant, the mass of excess ...
| PowerPoint PPT presentation | free to view
... 40 handles and 40 seats, how many tricycles can we make? ... Example 3.19 p. 114. A chemist set up a synthesis of phosphorus trichloride by mixing 12.0 g of ...
| PowerPoint PPT presentation | free to view
0.266 moles Br2 / 1 = 0.266 limiting. 42.5 grams Br2 1 mol Br2 2 mol KBr 119 g KBr ... 1 160 g Br2 1 mol Br2 1 mol KBr = 63.2 g KBr. When the reaction is done...
| PowerPoint PPT presentation | free to view
Stoichiometry: the study of the quantitative relationships between amounts of ... 100%, the reaction is very efficient (most of the reactants are converted to ...
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: Dan McCorkle Last modified by: Dan McCorkle Created Date: 6/9/2004 1:02:15 PM Document presentation format
| PowerPoint PPT presentation | free to download
substance, write the correct number of moles of substance s below the ... that the contents of the first two containers are combined in the third container. ...
| PowerPoint PPT presentation | free to view
To understand this concept, let's suppose you were an elf working for Santa ... of red to white is 1:1. Time is ticking and you find that you have 24 red stick ...
| PowerPoint PPT presentation | free to view
What happens to energy in during an exothermic reaction? Does a substance feel hot or cold during an exothermic reaction? Energy is released ...
| PowerPoint PPT presentation | free to view
420g NH3. How about something a little easier to follow???? Like. ... Instead of using the molar ratio you use volume ratio and solve for volume ...
| PowerPoint PPT presentation | free to view
Chemical Bonds and Energy. 7.3 Energy Changes in Reactions. AMDG ... There does not always have to be a bang, like in fireworks. 7.3 Energy Changes in Reactions ...
| PowerPoint PPT presentation | free to view
Transition metals make good heterogeneous catalysts because of their 4s and 3d electrons. A catalyst speeds up a reaction because it decreases the enthalpy change for ...
| PowerPoint PPT presentation | free to download
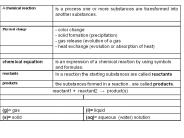
A chemical reaction is a process one or more substances are transformed into another substances. Physical change - color change - solid formation (precipitation)
| PowerPoint PPT presentation | free to download
Combustion will react the gasoline with oxygen to produce carbon ... Reaction Stoichiometry Assumptions. Reaction goes 100% to completion. No Side Reactions ...
| PowerPoint PPT presentation | free to view
Review for. Mass-Mass Limiting Reactant Test. 1. 2. 3. 3. 4. 5. 6. 7. ...
| PowerPoint PPT presentation | free to view
Collect Data from Determining the Percent Yield Lab. Clean up materials ... Did you consider the fact that CuSO4 is a hydrate? How does this alter your conclusions? ...
| PowerPoint PPT presentation | free to view
WRITING AP EQUATIONS AP equation sets are found in the free-response section of the AP test. You are given three sets of reactants and you must write balanced net ...
| PowerPoint PPT presentation | free to download
CHEMICAL REACTIONS Reactants: Zn + I2 Product: Zn I2
| PowerPoint PPT presentation | free to view
Synthetic planning (Retrosynthesis) Work Backwards .. Trace the reactions sequence from the desired product back to ultimate reactants. Starting reactant
| PowerPoint PPT presentation | free to download
Reactant concentrations start high and decrease as the reaction proceeds. ... The Reactant and Product concentrations are constant ...
| PowerPoint PPT presentation | free to download
Reaction Rate Chapter 17.1 Reaction Rate As time passes [reactant] decreases [product] increases Reactants Products Collision Theory A reaction ...
| PowerPoint PPT presentation | free to view
III Equation Problems A What does an equation represent? Represents chemical change Made up reactants and products Reactants starting materials
| PowerPoint PPT presentation | free to download
Reactions and Stoichiometry Chapters 11-12 Reactions Reactants Products Balancing Reactions Reactions must maintain conservation of mass, charge, and energy Reactants ...
| PowerPoint PPT presentation | free to download
REVERSIBLE REACTIONS A reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously
| PowerPoint PPT presentation | free to view
Stoichiometry Chemistry I: Chapter 12 Chemistry IH: Chapter 12 Limiting Reactant To find the limiting reactant, must try all of the reactants to calculate how much of ...
| PowerPoint PPT presentation | free to download
Reaction Rates Notes#12 Chapter 17 Reaction Processes & Rates Reactions are written in simplified forms-showing reactants turning into products reactants products ...
| PowerPoint PPT presentation | free to download
Chemical Reactions & Balancing Equations Chemical Reactions 2 Parts: Reactants: substances at the start Products: substances at the end The reactants turn into the ...
| PowerPoint PPT presentation | free to view
Limiting & Excess Reactants How do you know which one is which? What does limiting & excess mean? Limiting Reactant - the reactant that runs out first in a chemical ...
| PowerPoint PPT presentation | free to download
Directions for Foldable Define: Photosynthesis What are the reactants and products for Light Dependent Reactions? What are the reactants and products for Light ...
| PowerPoint PPT presentation | free to download
Stoichiometry Chapter 9 Limiting reagents In Stoichiometry: We work in moles. We cannot calculate grams of product from grams of reactant Step 1: Grams of reactant to ...
| PowerPoint PPT presentation | free to view
Chapter 4 Reactants: Zn(s) + I2(s) Product: ZnI2(s) Chemical Equations Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al(s) + 3 ...
| PowerPoint PPT presentation | free to download
Exp 19A: Oxidation-Reduction Reactions Oxidation-Reduction (Redox) reactions Net movement of electrons from one reactant to the other Movement from reactant with less ...
| PowerPoint PPT presentation | free to download
Determine the energies of the reactants, intermediates, ... Vary dihedral angles. Lining Up the Reactants* HF / 6-31 G(d) R = 3 Angstroms. E = -648.6805142 H ...
| PowerPoint PPT presentation | free to download
Reaction Rates Reaction Rate: The change in the concentration of a reactant or a product with time (M/s). Reactant Products aA bB Reaction Rates Consider the ...
| PowerPoint PPT presentation | free to download
Reaction Rates Reaction Rate: The change in the concentration of a reactant or a product with time (M/s). Reactant Products aA bB Reaction Rates Consider the ...
| PowerPoint PPT presentation | free to download
Arrhenius equation and transition state theory ... to the concentration of reactants raised ... The reactants must engage in an encounter (collision) event. ...
| PowerPoint PPT presentation | free to view
Classifying Reactions Synthesis Two or more reactants One product General Formula: A + B AB Decomposition One reactant Two or more products General equation ...
| PowerPoint PPT presentation | free to download
Drill: Draw & Name Reactants & Products of: Oxidations of 1-butanol in 2 steps:
| PowerPoint PPT presentation | free to download
... amounts of starting materials (reactants) the percent yield of the ... The reactant determining the amount of product generated is called the limiting reactant. ...
| PowerPoint PPT presentation | free to view
Balancing Chemical Reactions Steps to Success in Balancing Chemical equations Figure out whose a reactant and whose a product Write the formulas for the reactants on ...
| PowerPoint PPT presentation | free to download
If given mass of reactants for products, convert to moles first, then use the table. Limiting reactant problems Distinguish between what you start with and what reacts.
| PowerPoint PPT presentation | free to download
mole products - # mole reactants. Substitution for Kp. Kp = Kc(RT ... 2) The removal of reactants: this is 'bad' and will shift equilibrium toward the ...
| PowerPoint PPT presentation | free to view
Determining the limiting reagent. The masses of all reactants are given. must convert grams of each reactant into moles. make a ratio of the moles of each
| PowerPoint PPT presentation | free to download
Chemical kinetics The rate of a chemical reaction is dependent on: reactant concentrations state of reactants (solid, liquid, powder, etc.) temperature (e.g., eggs ...
| PowerPoint PPT presentation | free to download
The change in concentration of a reactant or product over time. Number of Molecules. of Reactants and Products vs Time. Reaction Rates and Stoichiometry. 13.1. aA ...
| PowerPoint PPT presentation | free to view
Rate of reaction is based on one component (reactant or product) of the reaction: ... reaction under the same conditions, varying only the concentrations of reactants ...
| PowerPoint PPT presentation | free to view
2. For each reactant, calculate the amount of product formed. 3. Smallest answer indicates: ... the limiting and excess reactants. How many liters of hydrogen ...
| PowerPoint PPT presentation | free to view
Predicting the Products of Chemical Reactions. Decomposition: Reactant: 1 compound ... Reactants: Two compounds. Products: Two different compounds, one of which ...
| PowerPoint PPT presentation | free to view
Plan: It is a limiting-reactant problem because the amounts of two. reactants are given. ... 1 mole H2. Percent Yield / Limiting Reactant Problem - I ...
| PowerPoint PPT presentation | free to view