Abstract PowerPoint PPT Presentation
1 / 1
Title: Abstract
1
Applications of Two-Dimensional NMR to the Study
of Cobalt(III) Complexes Containing
Tris(2-aminoethyl)amine and Ethylenediamine Dr.
Mark McClure and Stephanie Baker University of
North Carolina at Pembroke Department of
Chemistry and Physics
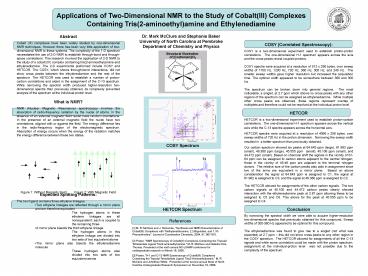
COSY (Correlated Spectroscopy) COSY is a
two-dimensional experiment used to establish
proton-proton correlations. The one-dimensional
H-1 spectrum appears across the axis and the
cross peaks reveal coupled protons. COSY spectra
were acquired at a resolution of 512 x 256 bytes,
over sweep widths of 1700 Hz, 1200 Hz, 720 Hz,
360 Hz, 300 Hz, and 240 Hz. The smaller sweep
widths gave higher resolution but increased the
acquisition time. The optimal width appeared to
be somewhere between 360 and 300 Hz. The
spectrum can be broken down into general regions.
The most noticeable, a singlet, at 2.7 ppm which
shows no cross peaks with any other regions of
the spectrum can be assigned as ethylenediamine.
While multiple other cross peaks are observed,
these regions represent overlap of multiplets and
therefore could not be resolved at the individual
proton level. HETCOR HETCOR is a
two-dimensional experiment used to establish
proton-carbon correlations. The one-dimensional
H-1 spectrum appears across the vertical axis
while the C-13 spectra appears across the
horizontal axis. HETCOR spectra were acquired
at a resolution of 4096 x 256 bytes, over sweep
widths of 720 Hz in the proton dimension.
Narrowing the sweep width resulted in a better
spectrum than previously obtained. Our carbon
spectrum showed six peaks at 64.643 ppm (large),
61.962 ppm (small), 46.380 ppm (large), 45.555
ppm (small), 45.106 ppm (small), and 44.472 ppm
(small). Based on chemical shift the signals in
the vicinity of 61-64 ppm can be assigned to
carbon atoms adjacent to the central nitrogen
those in the vicinity of 45-46 ppm are adjacent
to the terminal nitrogen donors. The relative
size of the carbon peaks also aids in assignment
since two of the arms are equivalent in a mirror
plane. Based on above consideration the signal
at 64.643 ppm is assigned to C1, the signal at
61.962 is assigned to C3, and the signal at
46.380 ppm is assigned to C2. The HETCOR allowed
for assignments of the other carbon signals. The
two carbon signals at 45.106 and 44.472 carbon
peaks clearly showed interaction with the
ethylenediamine peak at 2.87 ppm allowing these
to be assigned to C5 and C6. This allows for the
peak at 45.555 ppm to be assigned to
C4. Conclusion By narrowing the spectral width
we were able to acquire higher-resolution
two-dimensional spectra that previously obtained
for this compound. Sweep widths of 300-360 Hz
appeared to be optimal for this compound. The
ethylenediamine was found to give rise to a
singlet (not what was expected) at 2.7 ppm this
did not show cross peaks to any other region in
the COSY spectrum. The HETCOR allowed for
assignments of all the C-13 signals and while
some correlation could be made with the proton
spectrum, assignment at the individual-proton
level was not possible due to the complexity of
the spectrum.
- Abstract
- Cobalt (III) complexes have been widely studied
by one-dimensional NMR techniques. However there
has been very little application of
two-dimensional NMR to these systems. The
complexity of the 1-D spectrum necessitates the
use of 2-D NMR to establish through-bond and
through-space correlations. This research
involved the application of 2-D NMR to the study
of a cobalt (III) complex containing
tris(2-aminoethyl)amine and ethylenediamine. The
2-D experiments preformed include COSY and
HETCOR. The COSY, which shows through-bond
interactions, did not show cross peaks between
the ethylenediamine and the rest of the spectrum.
The HETCOR was used to establish a number of
proton-carbon correlations and aided in the
assignment of the C-13 spectrum. While narrowing
the spectral width produced higher-resolution
two-dimensional spectra than previously obtained,
its complexity prevented analysis of the spectrum
at the individual-proton level. - What is NMR?
- NMR (Nuclear Magnetic Resonance) spectroscopy
involves the absorption of radio-frequency
radiation by the nuclei of atoms. In the absence
of an external magnetic field nuclei have random
orientations. In the presence of an external
magnetic field the nuclei have two orientations
aligned with or against the field. The energy
difference lies in the radio-frequency region of
the electromagnetic spectrum. Absorption of
energy occurs when the energy of the radiation
matches the energy difference between these two
states. - Expected Splitting Patterns
Structural Illustration of Co(tren)enCl3
COSY Spectrum
Figure 1 Without Magnetic Field
Figure 2 With Magnetic Field
HETCOR Spectrum
References 1 M. R. McClure and J. Holcombe,
"Synthesis and NMR Characterization of
Cobalt(III) Complexes with Triethylenetetramine,
2,2-Bipyridine, and 1,10-Phenanthroline". Journal
of Coordination Chemistry, 2004, 57,
907-915.2 Poster, "NMR Spectroscopy of
Cobalt(III) Complexes Containing the Tripodal
Tetradentate Ligand Tris(2-aminoethyl)amine." M.
R. McClure and Natasha Oris-Thomas. Presented at
the ninth annual NC-LSAMP conference for
undergraduate research on March 18, 2005. 3
Poster, "H-1 and C-13 NMR Spectroscopy of
Cobalt(III) Complexes Containing the Tripodal
Tetradentate Ligand Tris(2-Aminoethyl)amine. M.
R. McClure and Johnithan White. Presented at the
second annual State of North Carolina
Undergraduate Research Symposium on November 18,
2006.