Using The Periodic Table PowerPoint PPT Presentation
Title: Using The Periodic Table
1
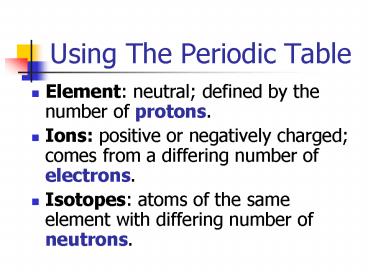
Using The Periodic Table
- Element neutral defined by the number of
protons. - Ions positive or negatively charged comes from
a differing number of electrons. - Isotopes atoms of the same element with
differing number of neutrons.
2
Using The Periodic Table
- Symbol, name, number of protons (all of these are
related). - Number of electrons in a neutral atom or in ion.
- Charges for common ions in compounds.
- Metals and non-metals.
- Formulas of some ionic compounds.
- Naming simple compounds.
3
Get these from the periodic table (except for
silver)!
To Know for the Exam
4
Know that the Roman numeral tells you the charge
To Know for the Exam
5
AmmoniumNitrateSulfateHydroxidePhosphateCarbo
nate
To Know for the Exam
6
To Know for the Exam
7
Question 1
- Which of the following is the correct name for
the compound with the formula MgF2? - a) magnesium (II) fluoride
- b) magnesium difluoride
- c) magnesium fluoride
- d) magnesium fluorite
- e) magnesium fluorate
8
Question 2
- Which of the following is named incorrectly?
- a) PCl3 phosphorus trichloride
- b) KCl potassium (I) chloride
- c) CuO copper (II) oxide
- d) Cu2O copper (I) oxide
- e) CO carbon monoxide
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.