Introduction to the Chemistry of NitrogenContaining Compounds: Amines - PowerPoint PPT Presentation
1 / 79
Title:
Introduction to the Chemistry of NitrogenContaining Compounds: Amines
Description:
A nitrogen-based link is vital in the polymeric molecules known as ... carbon-nitrogen bond distance in aniline slightly shorter than that in methylamine? ... – PowerPoint PPT presentation
Number of Views:267
Avg rating:3.0/5.0
Title: Introduction to the Chemistry of NitrogenContaining Compounds: Amines
1
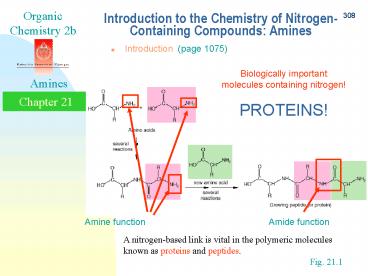
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- Introduction (page 1075)
Biologically important molecules containing
nitrogen! PROTEINS!
2
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- Introduction (page 1076)
Biologically important molecules containing
nitrogen! ALKALOIDS
3
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- Introduction (page 1076)
IMPORTANT HARD DRUGS ARE AMINES!!!
Amines!
Some alkaloids-nitrogen containing molecules with
great biological activity. Fig. 21.2
4
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1076)
Substituted amines. Fig. 21.3
5
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1076)
Examples
A naming scheme for primary, secondary and
tertiary amines. Fig. 21.3
6
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1077)
Name these amines!
A naming scheme for primary, secondary and
tertiary amines. Fig. 21.4
7
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1076)
A naming scheme for primary, secondary and
tertiary amines. Fig. 21.4
8
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1076)
Naming amines as substituted alkanes necessary
for more complex molecules!
Still another method for naming amines. Fig. 21.6
9
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1076)
Name these amines!
10
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.1 Nomenclature (page 1077)
Name these amines!
Still another method for naming amines. Fig. 21.6
11
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1080)
Why shorter than C-C and longer than C-O?
The carbon-nitrogen bond in amines is shorter
than a normal carbon-carbon bond. Fig. 21.14
12
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1080)
If true what could be the bond distance of CH3-F?
See 17.2 for reasoning!
The carbon-nitrogen bond in amines is shorter
than a normal carbon-carbon bond. Fig. 21.14
13
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1080)
Problem 21.1 Why is the carbon-nitrogen bond
distance in aniline slightly shorter than that in
methylamine?
14
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1081)
Sp3 hybridization at nitrogen and thus pyramidal!
Some bond angles in simple amines Fig. 21.16
15
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1081)
Why not exactly 109.5o?
Some bond angles in simple amines Fig. 21.16
16
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1081)
What happens here? Why not for carbon?
Amine inversion interconverts enantiomeric
amines Fig. 21.17
17
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1082)
Amine inversion has a planar transition
state. Fig. 21.18
18
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1082)
Amine inversion has a planar transition
state. Fig. 21.18
19
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1082)
What if the amine is chiral?
20
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1082)
Problem 21.4 In contrast to simple amines,
aziridines (three-membered rings containing a
nitrogen) can often be separated into
enantiomers. For example, the activation energy
for the inversion of 1,2,2-trimethylaziridine is
about 18.5 kcal/mole, much higher than for simple
amines. Explain.
Fig. 21.20
21
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1083)
replace by phenyl
Problem 21.5 If there is a phenyl group attached
to the nitrogen atom of the aziridine, the
barrier to inversion decreases.
22
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1083)
replace by phenyl
Problem 21.5 If there is a phenyl group attached
to the nitrogen atom of the aziridine, the
barrier to inversion decreases. Explain.
23
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.2 Structure and Physical Properties of
Amines (page 1085) - Decomposing fish owes its characteristic
unpleasant nature to amines which are liberated
in this process.
Problem 21.6 Many people use lemon when eating
fish. This custom is a carryover from the days
when it was difficult to preserve fish, and the
lemon acted to diminish the unpleasant odor (if
not the decomposition). Lemons contain 5-8
citric acid and this contributes to their soure
taste. Explain why lemon juice is effective at
reducing the odor of fish.
24
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1086)
Ammonia acting as a Brønsted base and as a
nucleophile Fig. 21.24
25
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1087)
Ammonium ions with high pKa values are related to
strongly basic amines, and ammonium ions with low
pKa values are related to weakly basic
amines. Fig. 21.25
26
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1087)
The pKa values for some simple ammonium
ions Table 21.2
27
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1087)
The gas-phase acidity of ammonium ions. Figure
21.26
28
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1087)
The gas-phase acidity of ammonium ions. Figure
21.26
29
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1088)
In solution, amines are stabilized through
dipole-dipole interactions and hydrogen bonding.
Large groups attached to nitrogen will interfere
with these stabilized interactions. Figure 21.27
30
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1089)
Problem 21.8
Amines can be stabilized by other factors.
Explain the pKa data in Figure 21.28
Figure 21.28
31
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1089)
Why?
Amines are much stronger bases than alcohols and
the corresponding ammonium ions are much weaker
acids than oxonium ions. Fig. 21.30
32
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1090)
Problem 21.9 The stability of oxonium ions
depends on the nature of the negatively charged
counterion. Fluoroborate (BF4-) is an especially
favorable counterion. Why?
33
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1090)
?
?
Primary and secondary amines are weak Brønsted
acids. Fig. 21.30
34
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1090)
Primary and secondary amines are weak Brønsted
acids. Fig. 21.31
35
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1090)
Very strong bases such as alkyllithium reagents
can remove a proton from an amine to give an
amide ion. Bu
CH2CH2CH2CH3 Fig. 21.32
36
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.4 Acid and Base Properties of Amines (page
1090)
The amide ion is a far stronger base (and
nucleophile) than the parent amine. Fig. 21.33
37
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1090)
- 21.5a Alkylation of Amines
38
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1090)
- 21.5a Alkylation of Amines
39
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1090)
- 21.5a Alkylation of Amines
40
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5a Alkylation of Amines
The formation of an ammonium ion through
alkylation of a tertiary amine Fig. 21.35
41
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
42
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
Imine and enamine formation from reactions of
amines with carbonyl compounds. Fig. 21.36
43
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 780)
Reactions of carbonyl compounds to give
hemiacetals and hydrates are completely analogous
to carbinolamine formation. The usual sequence of
protonation, addition and deprotonation is
followed. Fig. 16.48
44
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 780)
Reactions of carbonyl compounds to give
hemiacetals and hydrates are completely analogous
to carbinolamine formation. The usual sequence of
protonation, addition and deprotonation is
followed. Fig. 16.48
45
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 781)
Loss of water leads to a resonance-stabilized
intermediate in all reactions. Fig. 16.49
46
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 782)
In the second two reactions, loss of a proton
leads to an imine or carbonyl compound.
Fig. 16.50
47
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
Some substituted imines formed from reactions of
substituted amines with carbonyl
compounds. Fig. 16.50
48
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 784)
In order to form an imine, there must be two
hydrogens on the starting amine. That is, the
amine must be primary (RNH2) or ammonia (NH3).
Fig. 16.52
49
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 782)
Examples! Page 783
Some substituted imines formed from reactions of
substituted amines with carbonyl
compounds. Fig. 16.50
50
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
More examples! Page 783
51
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5b Additions of Amines to Carbonyl Groups
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
What happens if no hydrogens are present on
N? (tertiary amine)
52
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
What happens if one hydrogen is present on
N? (secondary amine)
53
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
What happens if one hydrogen is present on
N? (secondary amine)
Deprotonation of an immonium ion to give an
enamine is exactly like deprotonation of a
carbocation to give an alkene. Fig. 16.55
54
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
Compare with normal carbocation!
Deprotonation of an immonium ion to give an
enamine is exactly like deprotonation of a
carbocation to give an alkene. Fig. 16.55
55
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
Compare with normal carbocation!
Deprotonation of an immonium ion to give an
enamine is exactly like deprotonation of a
carbocation to give an alkene. Fig. 16.55
56
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 785)
Compare with protonated carbonyl compounds!
See Chapter 18!!!
Similar process!!!
57
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 16.9 Additions of Amines to Carbonyl Groups
(page 786)
Explain Why dont immonium ions formed from
primary amines give enamines rather than imines?
58
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
Fig. 16.56 (Page 786)
59
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Basic (alkaline) compounds extracted from natural
sources (plants) generally amines!
Usually chiral compounds and single enantiomers!
Some have powerful biological activity!
60
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Basic (alkaline) compounds extracted from natural
sources (plants) generally amines!
61
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Usually chiral compounds and single enantiomers!
62
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Some have powerful biological activity!
63
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Some alkaloids containing various nitrogen
heterocycles. Fig. 21.75
64
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1110)
Morphine
The structure of morphine. Fig. 21.76
65
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1110)
Find the differences!
66
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1110)
Reaction necessary for structure elucidation of
morphine, codeine etc.
A Hofmann elimination applied to
codeine. Fig. 21.77
67
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1110)
Problem 21.22 Write arrow formalisms for the
reactions of Figure 21.77
A Hofmann elimination applied to
codeine. Fig. 21.77
68
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1091)
- 21.5c Elimination Reactions of Amines
Hofmann elimination reactions of amines compared
to dehydration reactions. Fig. 21.39
69
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.5 Reactions of Amines (page 1093)
- 21.5c Elimination Reactions of Amines
Problem 21.12 Provide mechanisms for the
reactions of Figure 21.40
Fig. 21.40
70
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1111)
A non-natural derivative of morphine
What is the name of this well-known hard-drug?
Heroin!!!
Fig. 21.78
71
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1109)
Non-natural derivative of morphine
Morphine
Hardly addictive
Addictive!
Codeine and heroin are derivatives of
morphine. Fig. 21.78
72
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1111)
Non-natural derivative of morphine
Morphine
Problem 21.23 Unfortunately, morphine is easily
converted into heroin, even in a basement lab.
Suggest a way to achieve this conversion.
73
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1111)
From coca leaves an anesthetic.
Medicine pupil dilation inhibition of smooth
muscle spasms
Causes twilight sleep, a state of analgesia and
amnesia.
74
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1112)
Indole Alkaloids
neurotransmitter
75
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1112)
Indole Alkaloids
Powerful effects on brain and perception
(hallucination)
neurotransmitter
76
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.7 Alkaloids (page 1111)
Problem 21.25 The reactions in Figure 21.82 were
important in the synthesis of LSD. Provide
structures for the indicated compounds.
Fig. 21.82
77
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.8 Alkaloids (page 1113)
A powerful antimalarial agent
Quinine Fig. 21.83
78
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.8 Alkaloids (page 1114)
Problem 21.26 Outline mechanisms for the
following transfromations leading to quinine
(Fig. 21.84).
Fig. 21.84
79
Introduction to the Chemistry of
Nitrogen-Containing Compounds Amines
- 21.12 Additional Problems (page 1121)
- Problems 21.27 21.28 21.29 21.30 21.33 21.36
c,d,e,g,h.
Fig. 21.84