Amines - PowerPoint PPT Presentation
Title:
Amines
Description:
The presence of the amine group increases the reactivity of the aromatic ring ... Amine b.p are generally lower than alcohols. Soluble in water (upto 6 carbons) ... – PowerPoint PPT presentation
Number of Views:138
Avg rating:3.0/5.0
Title: Amines
1
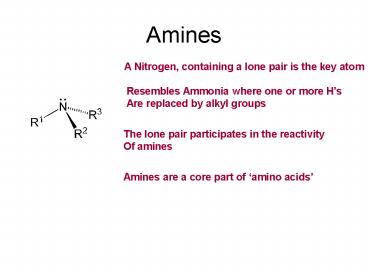
Amines
A Nitrogen, containing a lone pair is the key
atom
Resembles Ammonia where one or more Hs Are
replaced by alkyl groups
The lone pair participates in the reactivity Of
amines
Amines are a core part of amino acids
2
Amines (structure)
3
A fourth group is attached to the nitrogen
atom Using the lone pair. This is known as a
quaternary Ammonium ion
4
2-aminoethanol
2-aminopentane
TRIS buffer, used widely in biochemistry
5
Other examples
2-dimethylaminoethanol
2-methylaminoethanol
6
Amine Nomenclature (contd)
7
Aromatic amines
- The NH2 functional group is an amino group, and
when this group is a substituent, amino- is used
as a prefix - The presence of the amine group increases the
reactivity of the aromatic ring
8
Properties of amines Basicity
- Amines are electron pair donors
- Bronsted-Lowry bases or lewis bases
9
- Difference of boiling pts., similar molecular
weights, why ? - H-bonding significantly affects b.p
- Amine b.p are generally lower than alcohols
- Soluble in water (upto 6 carbons)
- Gaseous amines smell like ammonia, liquid amines
have a - A characteristic fishy smell!!
10
Will 3o amines have higher b.p than 1o amines ?
11
Heterocyclic Nitrogen compounds
Heterocycles are common in many naturally derived
products
12
(No Transcript)
13
Back to basicity of Amines
- Basicity depends on
- Availability of Lone pair of electrons
- - Is the protonated form of the amine
water-soluble? - - Substituent groups on the amine
Ions of compound Kb
Ammonia NH3 1.810-5 M
Methylamine CH3NH2 4.410-4 M
propylamine CH3CH2CH2NH2 4.710-4 M
2-propylamine (CH3)2CHNH2 5.310-4 M
diethylamine (CH3)2NH 9.610-4 M
14
Quarternary ions
Note heterocyclic ammonium ions are named by
using ium instead of -e