Physical vs' Chemical Changes - PowerPoint PPT Presentation
1 / 30
Title:
Physical vs' Chemical Changes
Description:
Balancing Chemical Equations. Do not change the formulas of reactants and products ... Balancing Chemical Equations. Mg(s) O2(g) MgO(s) Mg(s) O2(g) 2 MgO(s) ... – PowerPoint PPT presentation
Number of Views:74
Avg rating:3.0/5.0
Title: Physical vs' Chemical Changes
1
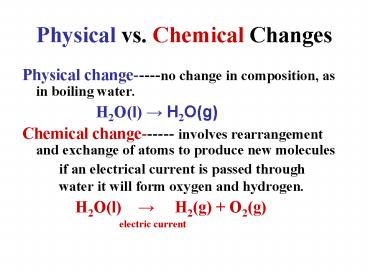
Physical vs. Chemical Changes
- Physical change-----no change in composition, as
in boiling water. - H2O(l) ? H2O(g)
- Chemical change------ involves rearrangement and
exchange of atoms to produce new molecules - if an electrical current is passed through
water it will form oxygen and hydrogen. - H2O(l) ? H2(g) O2(g)
- electric
current
2
Evidence of Chemical Reactions
- Permanent color / odor change
- Formation of a precipitate
- solid that forms when liquid solutions are mixed
- Gas evolution (bubbles)
- Transfer of electrons
- Energy changes
- container becomes very hot or cold
- emission of light
- Formation of water
3
Law of Conservation of Matter
- In a chemical reaction matter is neither created
nor destroyed. - This means that whatever elements are present
before a reaction takes place must still be
present after the reaction is over, though in
different combinations. - Total mass of reactants is equal to the total
mass of products.
4
Symbols Used in Chemical Equations
- symbols used to indicate state after chemical
- (g) gas (l) liquid (s) solid
- (aq) aqueous dissolved in water
- separates two or more formulas
- ? reacts to form products
- energy symbols used above the arrow for
decomposition reactions - D heat
- hn light
- shock mechanical
- elec electrical
5
Chemical Equations
- Reactants ? Products
- CH4(g) 2 O2(g) ? CO2(g) 2 H2O(g)
- CH4 and O2 are the reactants, and CO2 and H2O are
the products - the number in front of each substance tells us
the numbers of those molecules, formula units,
atoms in the reaction - called the Coefficients
- They have to be smallest integers (whole numbers)
6
Balancing Chemical Equations
- Do not change the formulas of reactants and
products - Use coefficients to balance all atoms, molecules,
formula units - Balance--------metals first
- nonmetals
- hydrogen
- oxygen last
- CaCl2(aq) Na3PO4(aq) ? Ca3(PO4)2s) NaCl(aq)
- 3CaCl2(aq) 2Na3PO4(aq) ? Ca3(PO4)2s) 6NaCl(aq)
7
Balancing Chemical Equations
- Mg(s) O2(g) MgO(s)
- Mg(s) O2(g) 2 MgO(s)
- 2 Mg(s) O2(g) 2 MgO(s)
- ammonia(g) oxygen(g) nitrogen monoxide(g)
- water(g)
- NH3(g) O2(g) NO(g) H2O(g)
- 2 NH3(g) 2.5 O2(g) 2 NO(g) 3 H2O(g) x 2
- 4 NH3(g) 5 O2(g) 4 NO(g) 6 H2O(g)
8
Classifying Reactions
9
Types of Reactions
- Combination
- N2(g) 3H2(g) ? 2NH3(g)
- Decomposition
- 2NaHCO3(s) ? Na2CO3(s) H2O(g) CO2(g)
- Combustion
- C3H 8(g) 5O2(g) ? 3CO2(g) 4H2O(g)
- Single replacement
- Zn (s) H2SO 4(aq) ? H 2(g) ZnSO4(aq)
- Double replacement
- AgNO3(aq) NaCl(aq) ? AgCl(s) NaNO3(aq)
10
Single ReplacementOxidation-Reduction (electron
transfer)
- Metal nonmetal ? ionic compound (salt)
- 2Na(s) Cl2 (g) ? 2NaCl(s)
- Nonmetal nonmetal ? covalent compound
- 2SO2(g) O2(g) ? 2SO3(g)
- CH4(g) 2O2(g) ? CO2(g) 2H2O(g)
- Metal acid or water ? H2(g) salt
- Zn(s) H2SO4(aq) ? H2(g) ZnSO4(aq)
- Ca(s) H2O(aq) ? H2(g) Ca(OH)2
11
Double Displacement precipitation reactions, gas
formation, water formation (acid-base)
- Precipitation Reactions
- AgNO3(aq) NaCl(aq) ? AgCl(s) NaNO3(aq)
- use solubility rules
- Gas formation reactions
- 2HCl(aq)Na2CO3(aq)?CO2(g)H2O(l) NaCl (aq)
- Acid-Base reactions (water formation)
- HCl(aq) NaOH(aq) ? NaCl(aq) H2O(l) heat
12
Dissociation
- when ionic compounds dissolve in water, the
anions and cations are separated from each other
- this is called dissociation - NaCl(aq) ? Na(aq) Cl-(aq)
- when compounds containing polyatomic ions
dissociate, the polyatomic group stays together
as one ion - AgNO3(aq) ? Ag(aq) NO3-(aq)
13
Electrolytes
- in strong electrolytes, all the electrolyte
molecules or formula units are separated into
ions - in nonelectrolytes, none of the molecules are
separated into ions - in weak electrolytes, a small percentage of the
molecules are separated into ions
14
Solubility RulesCompounds that are Generally
Soluble in Water
15
Solubility RulesCompounds that are Generally
Insoluble
16
Determine if Each of the Following is Soluble in
Water
- KOH
- AgBr
- CaCl2
- Pb(NO3)2
- PbSO4
17
MOLECULAR, IONIC NET IONIC EQUATIONS
- Molecular Equation
- 2KOH(aq) Mg(NO3)2(aq) 2KNO3(aq) Mg(OH)2(s)
- Ionic Equation
- 2K1(aq) 2OH-1(aq) Mg2(aq) 2NO3-1(aq)
2K1(aq) 2NO3-1(aq) Mg(OH)2(s) - Net Ionic Equation (cancel the spectator ions)
- 2OH-1(aq) Mg2(aq) Mg(OH)2(s)
18
Write the Molecular, Ionic and Net-Ionic
equations for the reaction below
- Molecular Equation
- 2HNO3(aq) Ca(OH)2(aq) ? Ca(NO3)2(aq) 2H2O(l)
- Ionic Equation
- 2H(aq) 2NO3-(aq) Ca2(aq) 2OH-(aq) ?
Ca2(aq) 2NO3-(aq) H2O(l) - Net Ionic Equation
- 2H1(aq) 2OH-1(aq) ? 2H2O(l)
- H1(aq) OH-1(aq) ? H2O(l)
19
Oxidation
- 1. Gain of oxygen 4Fe 3O2 ? 2Fe2O3
- The iron has been oxidized to iron III oxide
(rust). - 2. Loss of hydrogen
- CH3CH2OH O ? CH3CHO H2O
- The ethyl alcohol has been oxidized to
acetaldehyde. - 3. Loss of electrons
- Fe 2HCl ? FeCl2 H2
- The iron has been oxidized to the Fe2 ion,
losing two electrons (the same process takes
place forming Fe3)
20
Reduction
- 1. Loss of oxygen FeO CO ? Fe CO2
- Here the iron II oxide loses oxygen to form iron.
- 2. Gain of hydrogen
- CH3COCH3 2H ? CH3CH(OH)CH3
- Here acetone is reduced to isopropyl alcohol.
- Gain of electrons
- Mg Cl2 ? MgCl2
- Here the chlorine atoms are reduced to chloride
ions.
21
Oxidizers Reducers
- 2Al 3S ? Al2S3
- Oxidation never takes place without reduction
they occur simultaneously. (redox) - aluminum is oxidized to the aluminum ion so it
is called the reducing agent. - Sulfur is reduced to sulfide ion- so it is called
the oxidizing agent.
22
Oxidizers Reducers
- Oxidizing agents - Oxygen 21 of air, oxidizes
metals and nonmetals to oxides, and hydrocarbon
fuels to CO2 and H2O. Halogens. H2O2. Various
ions - ClO- MnO4- Cr2O72-. - Reducing agents - Hydrogen not found free,
secondary fuel when burned with oxygen also
reduces metal oxides to metals. Some metals and
carbon reduce other metal ores to metals.
23
Biological Oxidation Reduction
Energy is obtained from carbohydrates- C6H12O6
(aq) 6O2(g) ? 6CO2(g) 6H2O(l)
energy Each carbon has on average lost 2
hydrogens and gained 1 oxygen, so oxidation has
occurred. The reaction is reversed in
photosynthesis so this is reduction.
24
Energy Curves
25
Endothermic Exothermic Reactions
- Endothermic Reaction in a Cold Pack
- NH4NO3(s) H2O(l) 6.3 kcal ?NH4NO3 (aq)
- Exothermic Reaction in a Hot Pack
- CaCl2(s) H2O(l) ? CaCl2(aq) 18 kcal
26
Reaction Rates
- 1. Collision
- Orientation
- Temperature
- 4. Concentration (amount of reactant)
- Catalysis
- The rate (or speed) of a reaction is the amount
of reactant consumed or amount of product formed
in a certain period of time.
27
Temperature KE Distribution
28
Catalysis
29
Chemical Equilibrium
Many reactions can go in the reverse direction
(back reaction) and reform the reactants.
Reactant concentration decreases during a
reaction, therefore the forward rate
decreases. Products concentration increases
during a reaction, therefore the back rate
increases. When the rates of the forward and back
reactions are the same the system is in dynamic
equilibrium. For any one reaction the proportion
of reactants to products at a certain temperature
will always be the same.
30
Le Châteliers Principle
If a stress is put on a system in equilibrium it
will respond to minimize the stress to maintain
the balance. Example N2 3H2 ? 2NH3
Heat Add N2 and/or H2 - produces more product, a
shift to the right. Remove N2 and/or H2 - removes
product and forms more reactants - shift to the
left. Add NH3 - shifts to the left. Remove NH3 -
shifts to the right. Raise temperature (add heat)
- shifts to left. Lower temperature (remove) -
shifts to right.