ClinicalTrials.gov Registration: - PowerPoint PPT Presentation
1 / 1
Title:
ClinicalTrials.gov Registration:
Description:
ClinicalTrials.gov Registration: A Model for Determining the Responsible Party ... responsible party for registration and results reporting in ClinicalTrials.gov. ... – PowerPoint PPT presentation
Number of Views:29
Avg rating:3.0/5.0
Title: ClinicalTrials.gov Registration:
1
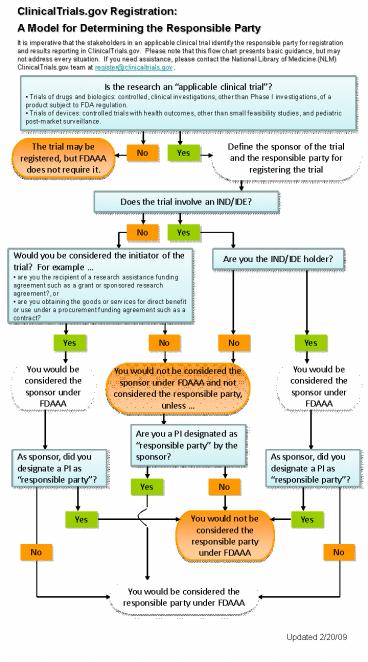
ClinicalTrials.gov Registration A Model for
Determining the Responsible Party It is
imperative that the stakeholders in an applicable
clinical trial identify the responsible party for
registration and results reporting in
ClinicalTrials.gov. Please note that this flow
chart presents basic guidance, but may not
address every situation. If you need assistance,
please contact the National Library of Medicine
(NLM) ClinicalTrials.gov team at
register_at_clinicaltrials.gov .
- Is the research an applicable clinical trial?
- Trials of drugs and biologics controlled,
clinical investigations, other than Phase I
investigations, of a product subject to FDA
regulation. - Trials of devices controlled trials with health
outcomes, other than small feasibility studies,
and pediatric post-market surveillance.
Define the sponsor of the trial and the
responsible party for registering the trial
The trial may be registered, but FDAAA does not
require it.
No
Yes
Does the trial involve an IND/IDE?
No
Yes
Are you the IND/IDE holder?
- Would you be considered the initiator of the
trial? For example - are you the recipient of a research assistance
funding agreement such as a grant or sponsored
research agreement?, or - are you obtaining the goods or services for
direct benefit or use under a procurement funding
agreement such as a contract?
No
No
Yes
Yes
You would not be considered the sponsor under
FDAAA and not considered the responsible party,
unless
You would be considered the sponsor under FDAAA
You would be considered the sponsor under FDAAA
Are you a PI designated as responsible party by
the sponsor?
As sponsor, did you designate a PI as
responsible party?
As sponsor, did you designate a PI as
responsible party?
No
Yes
You would not be considered the responsible party
under FDAAA
Yes
Yes
No
No
You would be considered the responsible party
under FDAAA
Updated 2/20/09