Protocol amendments - PowerPoint PPT Presentation
1 / 2
Title:
Protocol amendments
Description:
Title: Slide 1 Created Date: 5/2/2006 12:53:38 PM Document presentation format: A4 Paper (210x297 mm) Company: RJAH Orthopaedic Hospital Other titles – PowerPoint PPT presentation
Number of Views:31
Avg rating:3.0/5.0
Title: Protocol amendments
1
Funded by the MRC
AUTOLOGOUS CHONDROCYTE TRANSPLANTATION/IMPLANTATIO
N VERSUS EXISTING TREATMENTS
ISCRCTN 48911177
NEWSLETTER April 2008 Issue 14
- Protocol amendments
- Good news! After a lengthy process the
- MRC and ethics have approved the
- following changes to the protocol
- Extension
- An extension to the recruitment period
- of up to the end of 2009 to recruit at
- least 480 patients.
- Patella lesions
- Patella lesions can now be treated in
- the trial.
- OCDs
- Osteochondral defects can now be
- treated in the trial. Any OCDs
- involving more than 3 mm of bone loss
- must be grafted but the type and
- timing of bone graft is down to
- surgeons choice since there is a lack of
- evidence to support any one particular
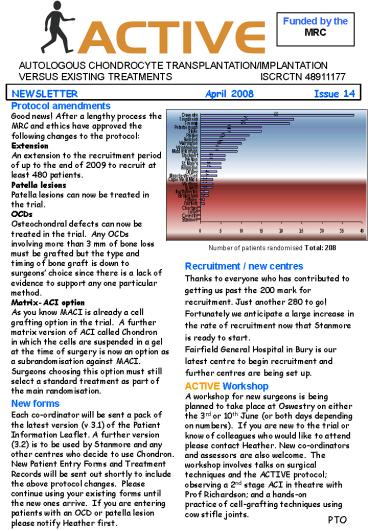
Number of patients randomised Total 208
Recruitment / new centres Thanks to everyone who
has contributed to getting us past the 200 mark
for recruitment. Just another 280 to go!
Fortunately we anticipate a large increase
in the rate of recruitment now that Stanmore is
ready to start. Fairfield General Hospital in
Bury is our latest centre to begin recruitment
and further centres are being
set up. ACTIVE Workshop A workshop for new
surgeons is being planned to take place at
Oswestry on either the 3rd or 10th June (or both
days depending on numbers). If you are new to
the trial or know of colleagues who would like to
attend please contact Heather. New
co-ordinators and assessors are also welcome.
The workshop involves talks on surgical techniques
and the ACTIVE protocol observing a 2nd stage
ACI in theatre with Prof Richardson and a
hands-on practice of cell-grafting techniques
using cow stifle joints.
New forms Each co-ordinator will be sent a pack
of the latest version (v 3.1) of the Patient
Information Leaflet. A further version (3.2) is
to be used by Stanmore and any other centres who
decide to use Chondron. New Patient Entry Forms
and Treatment Records will be sent out shortly to
include the above protocol changes. Please
continue using your existing forms until the new
ones arrive. If you are entering patients with
an OCD or patella lesion please notify Heather
first.
PTO
2
- Health economics
- An important aspect of ACTIVE is to evaluate the
cost effectiveness of ACI versus - standard treatments. We are fortunate that
recently Prof. Marilyn James and - Liz Stokes from Liverpool John Moores University
have taken on the role of Health - Economists for the trial. As a result we have
introduced some changes to the collection - of resource usage
- New Resource Usage forms have been sent out to
every centre. These are to be completed by
patients at 2/3, 6 12 months, then annually.
It is no longer collected at pre-op. We will
notify you when the database is ready to accept
these new forms. In the meantime please send
copies of completed forms to the trial office as
a backup. - Co-ordinators will be sent a new form and asked
to complete further details about hospital
resource usage using hospital notes (e.g. length
of stay in hospital additional procedures). This
new form is currently being piloted at Oswestry
but will be finalised and sent out soon.
UK-ICRS Meeting A UK-ICRS conference is planned
for the Autumn, to take place at Stanmore, led by
Prof. Dave Marsh and Prof. George Bentley date
to be confirmed. www.active-trial.org.uk The
latest version of the protocol can be downloaded
from the website under study documents along
with a summary of all the protocol amendments. A
hard copy will be sent to every centre in due
course. Best wishes, Heather Smith ACTIVE
Trial Manager Heatherj.smith_at_rjah.nhs.uk Tel
01691 404142
Happy at work in St. Olavs Hospital, Trondheim
from left to right Bente Johansen (co-ordinator)
Ingrid Solhjem (assessor) and Berit Wenaas (admin
assistant) looking very organised with their
ACTIVE files