Hcooh PowerPoint PPT Presentations
All Time
Recommended
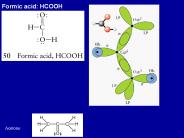
Formic acid: HCOOH. Acetone. Benzene C6H6. Resonance structures; each point corresponds to a CH ... Each C is sp2 hybridized, one of the sp2 forming a s-bond ...
| PowerPoint PPT presentation | free to download
Status of work. University of Oslo. Michael Gauss. Jan2000 Poet CTM2 ... hcooh (formic acid) -- ch3cooh (acetic acid) June: POET qfy. CO: nt: 29.04 73.02 ...
| PowerPoint PPT presentation | free to view
Electron transport chain. Lactate. CH3COHCOO- CH3COCOO- Pyruvate. Acetate. 2e ... Electron transport chain. HCOOH. HCOOH. CO2. Periplasm. Formate cycling' 2e- Formate ...
| PowerPoint PPT presentation | free to view
Annmarie G. Carlton, Gerald Gipson, Shawn Roselle, Rohit Mathur ... final gas phase partial pressure of SO2, NH3, HNO3, HCOOH, CO2. liquid conc. ...
| PowerPoint PPT presentation | free to view
Download free PDF Sample@ https://bit.ly/2XBmoF1 #ChemicalsAndMaterials #Chemicals #MarketAnalysis Formic Acid (also called methanoic acid) is a colorless liquid with a sharp odor. It is also the simplest carboxylic acid. The chemical formula is HCOOH or HCO2H. Formic acid is an environmentally acceptable and highly efficient organic acid.
| PowerPoint PPT presentation | free to download
2) Bronsted- Lowry: proton ( H ) donor. NH3(g) HCl(g) == NH4 ... solution of formic acid, HCOOH. Ka = 1.77 x 10-4. 12. HCOOH(aq) === H (aq) COOH1-(aq) ...
| PowerPoint PPT presentation | free to view
Carboxylic acids are acidic. ... oleic acid / H Methanoic acid, HCOOH as a reducing agent - Methanoic acid molecule, has both ...
| PowerPoint PPT presentation | free to view
Autooxidation of phospholipids are considered as responsible for the oxidized flavor: ... Methanethiol Ethylene Formic Acid. Strecker degradation. H2O. H2O HCOOH ...
| PowerPoint PPT presentation | free to view
Biogeochemical Cycling. Chapter 14 Text. Cycling Carbon, ... Carbon cycling by methanogens, methanotrophs and methylotrophs. CO2 CH4 CH3OH HCHO HCOOH CO2 ...
| PowerPoint PPT presentation | free to view
The main steps of MS measurements. Generate ions of the ... Effect of hydrophobicity. of the cosolvent. HCL HCOOC CH3COOH ( amalikova et al. 2004, Anal. ...
| PowerPoint PPT presentation | free to view
Gemini Observatory (HI) Kenneth Hinkle. National Optical Astronomy Observatories ... H2 H3 NH3 CH4 C2H4 C6H CH3C3N. CO H2O C2H2 SiH4 CH3CN CH2CHCN HCOOCH3 ...
| PowerPoint PPT presentation | free to download
Acid: Substance that, when dissolved in water, increases the ... M solution of formic acid, HCOOH, at 25 C is 2.38. Calculate Ka for formic acid at this ...
| PowerPoint PPT presentation | free to view
What are the Bioethical implications of Your Future Research? ... FeS H2S(aq) CO2(aq) FeS2 H2O HCOOH G0 = -12 kJ mol-1 ...
| PowerPoint PPT presentation | free to view
Composition hydrogen (75%, helium 25%) small amounts of dust (solid) ... Methylamine CH3NH2, formic acid HCOOH (can form gylcine NH2CH2COOH, an amino acid) ...
| PowerPoint PPT presentation | free to view
It is important to appreciate how this 'simple' fact can ... Effect of hydrophobicity. of the cosolvent. HCL HCOOC CH3COOH ( amalikova et al. 2004, Anal. ...
| PowerPoint PPT presentation | free to view
22 questions from chapters 6-8. Practice exam available on the class web ... M NaOH and 500 mL of a solution of 0.100 M formic acid (HCOOH, Ka = 1.77 x 10-4) ...
| PowerPoint PPT presentation | free to download
1000 year anniversary of SN1006 this week! Correlator technology: Huge ... 2003 showed the distribution of HCOOH (greyscale) at 7 cm was very similar to ...
| PowerPoint PPT presentation | free to download
a system will maintain a relatively constant pH if it already contains a ... HCOOH(aq) = HCOO-(aq) H (aq) formic acid Na formate ...
| PowerPoint PPT presentation | free to view
Acid: Any compound that increase the number of hydronium ions when dissolved in water. ... Formic Acid HCOOH bee sting. Citric Acid H3C6H5O7 weak lemons. Bases ...
| PowerPoint PPT presentation | free to view
Lewis structures are diagrams that show how electron pairs ... The summary sheet mentions HCOOH, formic/methanoic acid, which is also in Jones and Atkins (ex. ...
| PowerPoint PPT presentation | free to view
Formic, HCOOH. 1.4 x 10-3. Chloroacetic, CH2ClCOOH. 6.8 x 10-4 ... Calculate the pH of a 0.0010 M solution of formic acid, HCO2H. HCO2H H2O HCO2- H3O ...
| PowerPoint PPT presentation | free to view
Download FREE Sample Report- http://bit.ly/2KUiJgs Formic acid (also called methanoic acid) is a colorless liquid with a sharp odor. It is also the simplest carboxylic acid. The chemical formula is HCOOH or HCO2H. Formic acid is an environmentally acceptable and highly efficient organic acid.Globally, the global production was 1015 K MT in 2016 and it will reach 1217 K MT in 2022. In addition, the production regions of Formic acid are mainly located in China, US, EU and India. China was the leader production regions, which achieved about 48% volume market share in 2016. In 2015, BASF bullied the first plant of formic acid in Geismar, Louisiana. For more visit here- http://bit.ly/2Xs1Dbq
| PowerPoint PPT presentation | free to download
Calcium Formate is the calcium salt of formic acid, HCOOH. The chemical formula is Ca(HCOO)2. It is used as leather tanning, animal feed additive, cement additive, silage treatment etc. It may be produced synthetically by reacting calcium oxide or calcium hydroxide with formic acid. In this report, statistics includes feed and industrial Grade. Scope of the Report: This report focuses on the Calcium Formate in Global market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This report categorizes the market based on manufacturers, regions, type and application. Get Sample Copy@ http://www.researchtrades.com/request-sample/1091282
Title: Aucun titre de diapositive Author: SCORDIA Last modified by: SCORDIA Created Date: 7/15/2005 11:26:31 AM Document presentation format: Affichage l' cran
| PowerPoint PPT presentation | free to download
Ethanolic fermentation 2 pyruvate + 2 NADH - 2 ethanol + 2 CO2 Recap of Fermentations, cont. Respiration Aerobic Respiration In aerobic respiration, ...
| PowerPoint PPT presentation | free to view
PTH Process Hurricane chemical co.,ltd. Tel: 886-3-2127720 Fax: 886-3-3524476 PTH ...
| PowerPoint PPT presentation | free to download
Chemistry 100 Acids and Bases
| PowerPoint PPT presentation | free to view
5.4 5.4.1 5.4.2 5.4.3 5.4.4
| PowerPoint PPT presentation | free to view
Acid & Base Ionization Constants A weak acid or base produces a reaction that only partially goes forward. ... (formic acid) and found it to be 2.38.
| PowerPoint PPT presentation | free to download
ASAM BASA Oleh : Widodo, S.Pd. SMA N I SUMBEREJO TANGGAMUS by : Widodo-Prokimia@telkom.net ASAM BASA Oleh : Widodo, S.Pd. SMA N I SUMBEREJO ...
| PowerPoint PPT presentation | free to download
At regular time intervals, a fixed volume of the mixture is pipetted into large volume of ice-water to quench the reaction. (DO NOT QUENCH by Using NaOH!)
| PowerPoint PPT presentation | free to download
403221 (carboxylic acid) . .
| PowerPoint PPT presentation | free to download
(e.g. linear, trigonal bipyramidal etc)? What are the bond angles and molecule shape? ... (molecule is. pyramidal) trigonal bipyramid (molecule is T-shaped) ...
| PowerPoint PPT presentation | free to view
Catalysis in industrial processes
| PowerPoint PPT presentation | free to download
CARBOXYLIC ACIDS CONTENTS Structure of carboxylic acids Nomenclature Physical properties of carboxylic acids Preparation of carboxylic acids Chemical properties of ...
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Author: NGUYEN THIEN Last modified by: CNTT Created Date: 3/14/2006 2:19:12 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to view
Acid-Base Equilibria Chapter 16 The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved ...
| PowerPoint PPT presentation | free to download
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 16 Acids and Bases John D. Bookstaver
| PowerPoint PPT presentation | free to view
Acid-Base and Solubility Equilibria Common-ion effect Buffer solutions Acid-base titration Solubility equilibria Complex ion formation Qualitative analysis
| PowerPoint PPT presentation | free to view
The concept of pH and pKa Lecture 3 Handout continued base dissociation constant (or Kb) a measure of basicity pKb is the negative log of Kb and related to the pKa by ...
| PowerPoint PPT presentation | free to download
... (-1)L 2 mm Quantum cascade laser (QCL) 248 nm KrF excimer Pulsed Laser Hyperbolic Paul trap Optical cavity Experimental setup IR laser source HITRAN MHz v, ...
| PowerPoint PPT presentation | free to download
Title: Balancing Redox Equations Using the Half-Reaction Method Modified from Holt Modern Chemistry Author: Mounds View Schools Last modified by
| PowerPoint PPT presentation | free to download
Title: 1 Last modified by: cc2 Created Date: 9/5/2002 5:31:38 AM Document presentation format:
| PowerPoint PPT presentation | free to download
Strong Acid-Weak Base and Weak Acid - Strong Base ... Polyprotic acid and bases. Titration of H2CO3 with a strong base. Indicators ...
| PowerPoint PPT presentation | free to download
ALMA's Window on Molecular Emission: Small Ions to Complex Prebiotics Al Wootten
| PowerPoint PPT presentation | free to download
Title: Chapter 15 Acids and Bases Author: John Bookstaver Last modified by: Emilcomp Created Date: 2/11/2005 5:10:16 AM Document presentation format
| PowerPoint PPT presentation | free to view
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 16 Acids and Bases John D. Bookstaver
| PowerPoint PPT presentation | free to download
Acid-Base Equilibria Chapter 16 The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved ...
| PowerPoint PPT presentation | free to view
Acid-Base Equilibria. Chapter 16 ... Consider mixture of CH3COONa (strong electrolyte) and CH3COOH (weak acid) ... Adding more acid creates a shift left IF ...
| PowerPoint PPT presentation | free to view
313 101 Chemistry I (Thermochemistry) . . 4511-1
| PowerPoint PPT presentation | free to view
Mindbender Thursday april 17 What is a burette used for? What is a titration? What is an equivalence point in a titration curve?
| PowerPoint PPT presentation | free to download
Calculation of pH after the addition of 5 mL of 0.340 M HCl solution: ... pH at the stoichiometric (equivalent) point: pH after the hydrolysis of formate anion: ...
| PowerPoint PPT presentation | free to view
Title: Acid-Base Equilibria and Solubility Equilibria Author: J. David Robertson Last modified by: Sherri A. McFarland Created Date: 8/5/2001 9:58:52 PM
| PowerPoint PPT presentation | free to download
Interaction energy of formic acid is 0.25 eV higher for Pt-NCNT then Pt-CNT composite ... cyclic voltammograms of formic acid oxidation over pt loaded catalyst ...
| PowerPoint PPT presentation | free to view
Chapter 16 Acids and Bases Some Definitions Arrhenius Acid: Substance that, when dissolved in water, increases the concentration of hydrogen ions.
| PowerPoint PPT presentation | free to view