Materials and Methods: PowerPoint PPT Presentation
1 / 2
Title: Materials and Methods:
1
LOWER DECOMPRESSION SICKNESS RISK IN PIGS
SUPPLIED WITH H2-METABOLIZING MICROBES DURING
DIVES IN H2. A. Fahlman, S.R. Kayar, W. Lin, and
W.B. Whitman. Naval Med. Res. Center, Bethesda,
MD 20889 and Dept. of Microbiology, Univ. of
Georgia, Athens, GA 30602.
354.1
Materials and Methods Animals -Yorkshire pigs
(Sus scrofa), castrated males, n34, body mass
range 17-22 kg Groups 1) Untreated controls 2)
Surgical controls injected with 60 mL saline
into caecum and large intestine 3) Treated
animals injected with varying volumes (12-83
mL) and activities (200- 2200 ?mol CH4/min)
of Methanobrevibacter smithii into the caecum and
large intestine Surgery performed under
anesthesia, abdomen opened to allow access to
caecum and large intestine. Animals studied
immediately after recovery. Dive
simulation -Chamber pressurized to 24 bar
(21.6-22.9 bar PH2, 0.3-0.5 bar PO2) for 3
hr. -Decompression rate 0.9 bar/min to 11 bar
animals observed for 1 hr for DCS - Euthanized in
chamber on confirmation of DCS or at end of
hour. Measurements -Chamber gases analyzed by
gas chromatography for H2, O2, He, N2, and
CH4 -CH4 output rate (?mol CH4/min) from the
chamber was used as an indicator of the CH4
production rate from pigs. -Severe symptoms
of DCS included walking difficulties, fore
and/or hind limb paralysis, falling,
convulsions.
Abstract Pigs supplied with H2-metabolizing
microbes (n14 18.9?0.9 kg) had a 40 lower
incidence of decompression sickness (DCS)
compared to control animals (n10 19.6?1.6 kg),
following exposure to elevated pressures of H2.
Animals received caecal injections of either
Methanobrevibacter smithii (12-83 mL, activity
200-2200 mmol CH4/min) or 60 mL of saline, 1-2 h
prior to experiments. To simulate a H2 dive,
animals were placed in a dry hyperbaric chamber,
compressed to 24 bar (21.6-22.9 bar H2, 0.3-0.5
bar O2) for 3 h, then decompressed to 11 bar at
0.9 bar/min, and observed for 1 h for severe
symptoms of DCS. Chamber concentrations of O2,
N2, He, H2, and CH4 were measured by gas
chromatography throughout the dive. The CH4
release rate in treated animals (9.7-23.4
mmol/min) was used to indicate the microbial
activity of reducing the tissue burden of H2.
Treated animals had a significantly lower DCS
incidence (Plt0.05) than control animals (6/14 vs.
7/10), and a significantly higher mean CH4
release rate (Plt0.001) of 14.4?3.6 mmol/min vs.
6.7?2.5 mmol/min. Increasing the washout rate of
the inert gas by metabolizing H2 decreased the
DCS risk in a pig model during H2 dives.
(Supported by NMRDC work unit 61153N
MR04101.00D-1103 animal use guidelines of NIH
Pub. 92-3415, 1992).
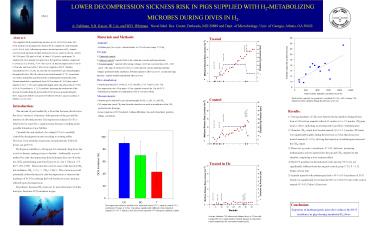
Treated
Total activity injected was positively correlated
(Plt0.01) with average CH4 output rate from
chamber during the last hour at 24 bar.
Control
Introduction The amount of gas breathed by a
diver that becomes dissolved in the divers
tissues is a function of the pressure of the gas
and the duration of elevated pressure.
Decompression sickness (DCS) is believed to be
caused by a rapid pressure decrease, resulting in
the possible formation of gas bubbles.
Currently the only method of avoiding DCS is to
carefully control the decompression rate
according to existing tables. However, even when
the ascent rates are meticulously followed,
divers can get DCS. Hydrogen is suitable as a
diving gas for extremely deep dives due to its
low density, making it easy to breathe.
Additionally, a novel method for safer
decompressions from hydrogen dives involves the
use of H2-metabolizing microbes (Kayar et al.,
Am. J. Physiol. 275 R677-682, 1998). These
microbes convert some of the dissolved H2 into
methane (4 H2 CO2 gt CH4 2 H2O). The
conversion could potentially reduce the time for
safe decompression or decrease the incidence of
DCS by reducing the body burden of excess inert
gas released upon decompression. Hypothesis
Increased H2 wash-out, by microbial removal of
the inert gas, decreases DCS incidence in pigs.
Results 1) Varying quantities of CH4 were
detected in the chamber during the last hour
at 24 bar from animals without M. smithii (6.6
2.9 ?moles CH4/min, mean stdev), indicating an
existing native gut flora of methanogens. 2)
Chamber CH4 output from treated animals (10.0
5.1 ?moles CH4/min) was significantly higher
during the last hour at 24 bar than from
non- treated animals (Plt0.01), showing that
injection of methanogens increased the CH4
output. 3) There was a positive correlation (
Plt0.01) between increasing methanogenic
activity injected into the pig and CH4 output
from the chamber, supporting a dose
response effect. 4) The DCS incidence in the
untreated control group (9/10) was not
significantly different from the surgical
control group (7/10, P gt 0.29, Fishers
Exact test). 5) Animals injected with methanogens
had a 43 (6/14) incidence of DCS which was
significantly lower than the 80 (16/20) DCS rate
of the control animals (Plt0.05, Fishers Exact
test).
Treated in He
Conclusion -Injection of methanogenic microbes
reduces the DCS incidence in pigs during
simulated H2 dives
Decompression sickness incidence for untreated
control (UC), surgical control (SC), and treated
(T) pigs at 24 bar. Incidence significantly
different from untreated animals (Plt0.05,
1-tailed ?2-test) Error bars represent 95
binomial confidence limits.
Average chamber CH4 release rate during dives to
24 bar with varying PH2 for a representative
treated animal, an untreated control animal and
for one treated animal in He.
2
Points of Contact NMRI-Principal
Investigator Dr. Susan Kayar, E-mail
kayars_at_nmripo.nmri.nnmc.navy.mil Phone(301)295-5
903 Research Assistant Andreas Fahlman, E-mail
fahlmana_at_nmripo.nmri.nnmc.navy.mil Phone(301)295
-5867