TACT Enrollment - PowerPoint PPT Presentation
1 / 4
Title:
TACT Enrollment
Description:
326 Kenneth Ganapini/ Venus Barney 4. 232 John Griffin/ Terry Mellinger 4 ... 238 Lawrence Miller/ Deanna Overbeck 1. 122 Harmony Reynolds/ Karen Hager 1 ... – PowerPoint PPT presentation
Number of Views:153
Avg rating:3.0/5.0
Title: TACT Enrollment
1
TACT LOGO
NIH
TACT Newsletter
April 2004
Our congratulations and kudos to Site 234! Dr.
Rajiv Chandra, Terry Murphy and Susan Hewitt from
Tru Med ED of Melbourne, Florida enrolled 8
patients in 7 days !!! What an example to follow!!
Message from the Principal Investigator
Gervasio A. Lamas, MD Since our last quarterly
Newsletter, we held our second Investigators and
Coordinators Meeting on March 25-28. It was a
huge success, with about 60 sites attending the
meeting in Miami Beach. Participants attended
lectures and received important hands-on
training. There was even time for sites to
demonstrate their talent at the TACT Talent show!
Congratulations to Dr. Alden and Ashley Steele
for winning first place for their Romeo, Juliet
and TACT performance! We now have over 80 sites
that can enroll patients! The enthusiasm
generated from the meeting has pushed enrollment
to 165. We have now surpassed our targets for
the month of April! Thanks to all the site
investigators and coordinators who helped make
this happen. A special congratulations goes to
Dr. Rajiv Chandras site who has enrolled 15
patients since the investigators meeting. Dr.
Shah is still our number 1 enroller, with 20
patients. We have learned from the successful
sites that you must approach all potential
patients in order to increase enrollment. I
challenge all of you to be our number one
site! We should all be optimistic that this
trial will be a success. However, without the
continued dedication and efforts from
Investigators and Coordinators we will never
reach our goals. To be successful, sites must
convey their belief in the importance of this
trial to patients. We still have a long way to go
before we reach our goal of enrolling 2372
patients. We all know this trial is difficult
and controversial. If you have any concerns about
any aspect of this trial, please contact us. We
are happy to address any issues you may have.
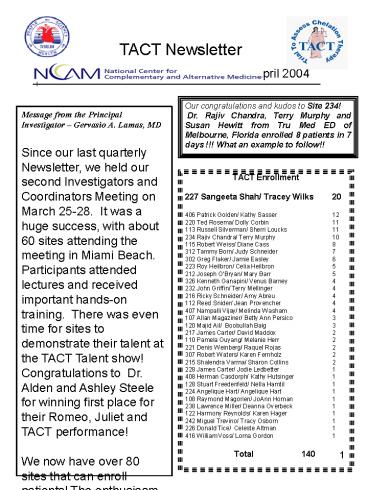
- TACT Enrollment
- 227 Sangeeta Shah/ Tracey Wilks 20
- 406 Patrick Golden/ Kathy Sasser 12
- 220 Ted Rosema/ Dolly Corbin 11
- 113 Russell Silverman/ Sherri Loucks 11
- 234 Rajiv Chandra/ Terry Murphy 10
- 115 Robert Weiss/ Diane Cass 8
- 312 Tammy Born/ Judy Schneider 7
- 302 Greg Flaker/ Jamie Easley 6
- 223 Roy Heilbron/ Celia Heilbron 5
- 212 Joseph OBryan/ Mary Barr 5
- 326 Kenneth Ganapini/ Venus Barney 4
- 232 John Griffin/ Terry Mellinger 4
- 216 Ricky Schneider/ Amy Abreu 4
- 112 Reed Snider/ Jean Provencher 4
- 407 Nampalli Vijay/ Melinda Washam 4
- 107 Allan Magaziner/ Betty Ann Persico 3
2
TACT Newsletter Continued
- QUEST Laboratories
- As a reminder, only laboratory tests required by
the TACT study protocol are to be sent to the
Quest laboratories. Any ancillary tests are to
be performed at your local laboratory, using
their requisitions. The routine laboratory
examinations for this study are identified on
page 45 of the protocol or behind the Laboratory
Tab in the TACT Study Manual. If patients are
unable to proceed with their infusions due to
laboratory delays, the repeat safety bloods
should continue to be sent to Quest.
- Pharmacy Reminder
- A way to communicate with the pharmacy is right
at your figure tips. Use the Message tab in
TrialMaster and compose a note. Simply select
compose and open the address book. The
pharmacy is listed second, click this and set
the priority to HIGH. In the subject line,
specify infusion date change. In this way the
pharmacy is alerted to your immediate need. This
is especially useful if you need to change a
patients infusion date and are concerned whether
or not you will receive your infusion in time for
the next scheduled visit. - Visit Projector
- We hope that you have all taken advantage of the
Visit Projector that has been produced
especially for the TACT study. This program
allows you to see into the future, the
subsequent dates each patient will be infused.
This will help determine potential scheduling
conflicts for the site and the patient. This
information is on the TACT website.
Extra Forms To help C of extra study forms.
These forms can be found in the TACTNIH.COM
website, the Document Tab in TrialMaster or your
study manual. Website Reminder Please
remember to check the tact website for updates
frequently. As we receive letters from the DSMB
or other important information, it will be placed
in the TACT update section of the
website www.tactnih.com username
infusion Password tactnih
3
TACT Newsletter Continued
- All things being EQOL
- Question If a patient is randomized to EQOL,
- does he/she have to participate?
- Yes, if someone is randomized to EQOL follow
- up, it is required by the protocol that he/she
- participate in EQOL. There will be 1,000
patients - participating in this important substudy. This
is an - opportunity for them to provide information that
- may determine how these treatments affect the
- quality of life.
- Patients randomized to EQOL will answer only
- three questionnaires during the entire follow-up
- period. These will be done at 6 months, 1 year
- and 2 years. Please communicate this to your
- patients so they will understand the importance
of - participating in the survey. If there is a
problem
Hypocalcemia When Ca is lt 9 mg/dL take the
following steps 1. Increase infusion
administration time from three hours to 4 - 5
hrs (100 to 125 cc/ hr). 2. Re-draw calcium
before the next infusion 3. The abnormal calcium
value is considered an adverse event and
requires completion of an AE form If untreated,
hypocalcemia may cause intestinal cramps,
arrhythmias, and muscle spasms. If severe and
chronic, hypocalcemia may cause seizures and
respiratory arrest.
Extra Items to Have on Hand Calcium gluconate
for treatment of severe hypocalcemia, as ordered
by MD Heating pads for discomfort at IV
site Finger stick glucose monitors Oral and IV
glucose IV lasix O2 available
SPECIAL HINT It is best to use the antecubital or
proximal veins for the infusions rather than hand
veins.
- Herbal Supplements
- It has been noted on the iCRF that the section
for - recording Herbal Supplements also includes the
- listing of some nutritional supplements. To
- accommodate this fact, a slight modification
- to the wording will be made to this form. Thank
- you for your understanding regarding this issue
TACT Communication TACT (DCRI) Helpline
800-545-3853 Mt. Sinai 305-674-2794
DCRI 919-668-8253
4
TACT Newsletter Continued
Vitamin Accountability
- The vitamin accountability form has its home
under the Infusion 1 visit, since this is when
vitamins are first dispensed. There are currently
five spaces available for both tablets and
blister packs. It will generally take from 9 to
12 months to complete this first record,
depending on what has been provided to each
site/patient from the pharmacy. When the form has
been filled, you will need to add another Vitamin
Accountability form. This new form will also be
housed under Infusion 1, so you will always know
where it is! This will continue even through
maintenance and follow-up. - To maintain accurate dispensing records, make
sure that the patients are instructed to return
all bottles/tablets and gel-caps/blister packs.
Returned vitamins are to be counted and the
number remaining as well those that are
unaccounted for documented in the appropriate
space. You will need to calculate how many
tablets/gel-caps should have been taken by
counting the days from the date the drugs were
dispensed. - Unaccounted tablets/gel-caps are defined as lost
or discarded accidentally by the patient.
When vitamins are re-supplied, you should
calculate your patients' compliance to the
protocol prescribed directions. This compliance
percentage should be documented on your infusion
visit worksheet (source). If the percentage is
less than 85 talk with your patient to find out
why and provide counsel as to the importance of
taking the vitamins as prescribed. The compliance
percentage is calculated by count returned
pills (separate high and low dose) subtract
the number of pills returned from the number of
pills distributed this will yield ACTUAL pills
taken count the number of days between
distribution and resupply this is the total days
for vitamins to be taken for high dose, multiply
total count by 6 for low dose multiply total
count by 1 this will yield EXPECTED pills to be
taken divide the ACTUAL pills taken by the
EXPECTED pills taken and multiply by 100 to
obtain your compliance percentage.
If you have questions, please call your Regional
Coordinator at DCRI.