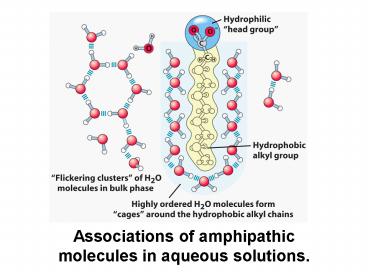
Associations of amphipathic molecules in aqueous solutions. - PowerPoint PPT Presentation
1 / 74
Title:
Associations of amphipathic molecules in aqueous solutions.
Description:
Associations of amphipathic molecules in aqueous solutions. Amino acids bear structural similarity to each other Phenylalanine Tyrosine Phenylalanine Leucine ... – PowerPoint PPT presentation
Number of Views:209
Avg rating:3.0/5.0
Title: Associations of amphipathic molecules in aqueous solutions.
1
Associations of amphipathic molecules in aqueous
solutions.
2
(No Transcript)
3
Ionic Mobilities in H2O at 25C.
4
(No Transcript)
5
Mean lifetime of a hydronium ion is 10-12 s
This makes proton transfer reactions (acid base
reactions) among the fastest in aqueous solutions.
6
Acid Base Chemistry
Conjugate acid
Conjugate base
HA H2O
H3O A-
H3OA-
K
H2O 55.5M
HAH2O
HA-
Ka KH2O
H3O H
HA
K dissociation constant is a measure of the
strength of an acid
7
Water as an acid
Conjugate acid
Conjugate base
H2O
H OH-
HOH-
K
H2O 55.5M
H2O
Kw KH2O
HOH-
Pure water contains equimolar hydroxide ions and
protons At 25ºC Kw 10-14 M2 H OH- 10-7
M
8
Henderson Hasselbach and pH
pH
-logH
H
Ka(HA/A-)
pH
-log Ka log (A-/HA)
pH
pKa log (A-/HA)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
Titration curve of a 1L solution of 1M H3PO4.
13
Thermodynamics
First Law Energy is conserved
?U Ufinal - Uinitial q - w
q heat absorbed w work done
?U 0 for any process that returns to its
initial state
Exothermic processes release heat Endothermic
processes gain heat
14
Enthalpy is defined as
H U PV
P pressure (constant) ?V volume
(insignificant)
?H ?U P?V
w often is zero in biological systems
?H ?U q - w
?H q
q heat transferred to/from the surroundings
15
Thermodynamics
Second Law Entropy increases
?Suniverse gt 0
16
2N equally probable ways of distributing them
N molecules of gas
Two bulbs of equal volumes connected by a
stopcock.
17
WL number of different ways of placing L of the
N molecules in the left bulb
N!
WL
L!(N-L)!
Probability WL/2N
For any N the most probable state is L N/2
(half the gas in the left bulb)
If N 1023 the probability that the of
molecules in the left and right bulbs differ by 1
molecule is 10 billion in 10-434
18
WL number of different ways of placing L of the
N molecules in the left bulb
N!
WL
L!(N-L)!
9 positions, 4 identical balls
W 987654321 126
(4321)(54321)
Only 4 out of 126 possible arrangements have 4
balls touching each other
Page 54
19
N!
W is approximately 107x1022 if the previous
experiment uses a mole of real gas
WL
L!(N-L)!
To make this more manageable entropy was
invented
S kB ln W
In a system where energy does not change a
spontaneous process has ?S gt 0
20
This does not mean that order cannot exist In a
localized system. It means that order can only
exist at the expense of surrounding
systems. Biology gains order by disordering the
nutrients that it consumes.
?Ssystem ?Ssurroundings ?Suniverse gt 0
21
Free Energy
G H - TS
?G ?H - T?S
?G 0 for a spontaneous process
22
Exergonic ?G lt 0 Spontaneous Endergonic ?G gt
0 Must input energy
23
Variation of Reaction Spontaneity (Sign of ?G)
with the signs of ?H and ?S.
24
How do we drive endergonic processes?
25
(No Transcript)
26
Greek lettering scheme used to identify the atoms
in the glutamyl and lysyl R groups.
27
An a-amino acid
28
(No Transcript)
29
a
a
a
a
Fischer Projection
Preferred representation
Glycine - The Simplest a-Amino Acid
30
L-a-alanine or (-)- a -alanine
Alanine
a
a
Ca
a
b
(S)-a-alanine
S counterclockwise
31
a-valine L-(-)-a-valine S-a-valine
Valine
g1
b
a
g2
a
Ca
a
32
a -leucine L-a-leucine (-) -a-leucine S-a-leucine
d1
Leucine
b
a
g
d2
a
Ca
a
33
Isoleucine2 chiral centers(2S,3S)-isoleucine
g2
d1
a
b
g1
a
a
34
Isoleucine2 chiral centers(2S,3S)-isoleucine
Ca
Cb
Both centers are S
35
Methionine is non-polar but S-atom is reactive
a-methionine L-methionine (-)-a-methionine S-methi
onine
b
g
e
d
a
a
36
Methionine is non-polar but S-atom is reactive
a-methionine L-methionine (-)-a-methionine S-methi
onine
a
Ca
37
Proline is a cyclic imino acid
a-proline L-proline (-)-a-proline S-proline
b
a
g
e
d
a
2
2
2
a
Ca
2
38
(No Transcript)
39
Large non-polararomatic
a-phenylalanine L-phenylalanine (-)-a-phenylalanin
e S-phenylalanine
e1
d1
b
a
a
g
z1
d2
e2
40
Large and non-polar
a-phenylalanine L-phenylalanine (-)-a-phenylalanin
e S-phenylalanine
Ca
a
41
Large and non-polar
a-tryptophan L-tryptophan (-)-a-tryptophan S-trypt
ophan
a
z3
e3
h2
d2
b
e2
a
z2
g
d1
e1
42
Large and non-polar
a-tryptophan L-tryptophan (-)-a-tryptophan S-trypt
ophan
Ca
a
43
a-tyrosine L-tyrosine (-)-a-tyrosine S-tyrosine
Uncharged Polar Amino Acids
a
d1
e1
b
a
g
z1
h
d2
e2
44
a-tyrosine L-tyrosine (-)-a-tyrosine S-tyrosine
Uncharged Polar Amino Acids
Ca
a
45
(No Transcript)
46
Uncharged Polar Amino Acids
a-serine L-serine (-)-a-serine S-serine
a
a
g
b
Ca
a
47
Uncharged Polar Amino Acids - cysteine is often
charged
a-cysteine L-cysteine (-)-a-cysteine R-cysteine
b
g
a
Ca
a
a
48
Uncharged Polar Amino Acids
a-asparagine L-asparagine (-)-a-asparagine S-aspar
agine
a
d1
b
a
g
d2
Ca
a
49
a-glutamine L-glutamine (-)-a-glutamine S-glutamin
e
Uncharged Polar Amino Acids
e1
b
a
g
d
a
e2
Ca
a
50
Threonine has 2 chiral centers(2S,3R)-threonine
b
a
g2
g1
a
a
51
Threonine has 2 chiral centers(2S,3R)-threonine
Ca
Cb
52
(No Transcript)
53
Charged amino acids
a-arginine L-arginine (-)-a-arginine S-arginine
h2
a
b
g
z
a
d
e
h1
a
Ca
54
a-lysine L-lysine (-)-a-lysine S-lysine
Charged amino acids
g
z
a
b
d
e
a
a
Ca
55
a-histidine L-histidine (-)-a-histidine S-histidin
e
Charged amino acids
d1
e1
b
a
g
a
e2
d2
a
Ca
56
(No Transcript)
57
Charged amino acids
a-glutamate L-glutamate (-)-a-glutamate S-glutamat
e
e1
a
b
g
a
d
e2
Ca
a
58
a-aspartate L-aspartate (-)-a-aspartate S-aspartat
e
Charged amino acids
d1
b
a
g
a
d2
a
Ca
59
Alanine Ala A Cysteine Cys C Glycine Gly G Histidi
ne His H Isoleucine Ile I Leucine Leu L Methionine
Met M Proline Pro P Serine Ser S Threonine Thr
T Valine Val V
Arginine Arg R Asparagine Asn N Aspartate Asp D Gl
utamate Glu E Glutamine Gln Q Lysine Lys K Phenyl
alanine Phe F Tryptophan Trp W Tyrosine Tyr Y
60
Non-standard encoded amino acids
Selenocysteine Sec, U
a
Pyrrolysine Pyl, O
a
61
Amino acids bear structural similarity to each
other
Asparate
Glutamate
d1
e1
b
b
g
a
a
g
d
d2
e2
Asparagine
Glutamine
d1
e1
b
a
g
b
a
g
d
d2
e2
62
Amino acids bear structural similarity to each
other
Cysteine
Selenocysteine
b
g
a
a
Threonine
Serine
b
a
a
g
b
g2
g1
63
Amino acids bear structural similarity to each
other
Tyrosine
d1
e1
b
a
g
z1
h
d2
e2
Phenylalanine
e1
d1
b
a
g
z1
d2
e2
64
Amino acids bear structural similarity to each
other
Histidine
Histidine
Arginine
Asparagine
Histidine
Histidine
Arginine
Glutamine
65
Amino acids bear structural similarity to each
other
Histidine
Tryptophan
66
Amino acids bear structural similarity to each
other
Phenylalanine Tyrosine
Phenylalanine Leucine
67
- Glutamate, glycine
- neurotransmitters
- D-serine
- neurotransmitter
- S-adenosylmethionine
- methyl transfer
68
Non-peptide amino acids
Page 77
69
Titration curve of glycine.
70
(No Transcript)
71
(No Transcript)
72
(No Transcript)
73
These values are the pKas of the free amino
acids in aqueous solution. As we shall see later
an aqueous solution may not represent reality
74
These values are the pKas of the free amino
acids in aqueous solution. As we shall see later
an aqueous solution may not represent reality
Hydrophobic pocket