Infrared Absorption PowerPoint PPT Presentation
1 / 26
Title: Infrared Absorption
1
(No Transcript)
2
Infrared Absorption In order to absorb IR
radiation, a molecule must undergo a net change
in dipole moment as a consequence of
its vibrational or rotational motion.
3
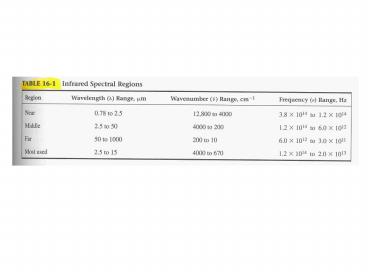
Rotational Transitions Corresponds to radiation
of 100 mm and greater (far IR) Gases discrete,
well-defined lines Liquids and Solids
Intramolecular collisions and interactions cause
broadening of lines to a continuum
4
Vibrational-Rotational Transitions Near and Mid
IR (0.78-15 mm) Gases series of closed spaced
lines corresponding to several rotational energy
states of each vibrational state. Liquids and
Solids Rotation is restricted, so
broadened vibrational peaks.
5
Molecular Vibrations Stretching continuous
change in interatomic distance along the axis of
the bond between two atoms Bending
characterized by a change in the angle between
two bonds. Four types scissoring, rocking,
wagging, and twisting
6
Harmonic oscillator (left) Follows Hookes Law
F -ky where F is restoring force and k is force
constant. Anharmonic oscillator (right) at
higher quantum numbers, smaller change in energy
leads to overtone lines at frequencies two to
three times the fundamental line.
7
- Possible Vibrations in Polyatomic Molecule
- Three coordinates fix a point in space, so a
molecule having N - Atoms has 3N degrees of freedom.
- To define motion of molecule
- Motion of entire molecule in space (three degrees
of freedom) - Rotational motion of entire molecule around its
center of - gravity (three more degrees of freedom)
- Motion of each atom relative to other atoms
- Therefore 3N-6 represents number of possible
vibrations. - Linear molecules cannot rotate about bond axis,
so 3N-5 vibrations. - These 3N-6 (or 3N-5) vibrations are each called a
normal mode.
8
Number of Observed Absorption Peaks Is usually
less than number of normal modes because 1)
There may not be a change in dipole from a
particular vibration, due to molecular
symmetry 2) Energies of two (or more) vibrations
is essentially identical 3) Absorption intensity
is so low its undetectable 4) Vibrational
energy is in a wavelength region beyond the range
of the instrument
9
Influences for Vibrational Coupling 1) Coupling
of stretchng vibrations occurs when common
atom. 2) Coupling of bending vibrations occurs
when common bond. 3) Coupling of a stretching and
a bending vibrations if stretching bond forms one
side of angle that varies in the bending
vibration. 4) Interacton greatest when coupled
groups have about the same energy. 5) Little
interaction between groups separated by two or
more bonds. 6) Coupling requires same symmetry
species.
10
Examples of Vibrational Coupling
11
Reflection Gratings All commercial dispersive
systems use reflection gratings rather than
prisms as dispersing elements. Advantages Better
resolution since little loss of radiant
energy. Linear dispersion. Resistant to attack by
water. Disadvantage Greater scattered radiation.
12
Infrared Detectors Thermal detectors typically
used -- response depends on heating effect of
radiation.
13
Advantages of FTIR Enhanced signal-to-noise Rapid
scanning High resolution (lt0.1 cm-1) Accurate
and reproducible frequency determinations Larger
energy throughput Free from problems of stray
radiation
14
Areas where FRIT particularly useful High-resolut
ion needed for gaseous mixtures with
superposition Of vibrational and rotational
bands Samples with high absorbances Substances
with weak absorption bands Fast scanning
kinetic studies, chromatography detection Small
sample size Reflection spectra IR emission
spectra
15
Comparison of Double-beam and Single-beam Systems
16
Schematic of Double-Beam System
Heavy line mechanical linkage Light line
electrical linkage Dashed line radiation path
17
(No Transcript)
18
Solvent Ranges for IR
19
Group frequency region determine functional
groups Fingerprint region fine, specific
structure
20
(No Transcript)
21
Deviations from Beers Law Because of narrow
absorption bands, deviations from Beers Law are
more common with IR than UV-Vis. Low intensity
sources and low sensitivities require bandwidths
on same order as widths of absorption peaks.
22
Limitations to Quantitative IR Deviations from
Beers Law. Complexity of spectra (overlap of
absorption peaks). Narrowness of peaks and
effects of stray radiation. Narrow cells may
lead to analytical uncertainities.
23
Sample spectra from C8H10 Isomers
24
Comparison of absorption spectrum (top)
and reflectance spectrum (bottom)
25
Near-IR Usually C-H, N-H, and O-H
bonds. Quantitative determination of water,
proteins, low-MW hydrocarbons, fats in food and
agricultural products. Near-IR reflectance used
for routine quantitative determination of
constituents in finely ground solids.
26
Far IR Useful for inorganics due to stretching
and bending vibrations of bonds between metal
atoms and inorganic or organic ligands (usually
at frequencies less than 650 cm-1).