Infrared Absorption Spectroscopy - PowerPoint PPT Presentation
1 / 44
Title:
Infrared Absorption Spectroscopy
Description:
... temperatures between 1200 and 2200 K ... Times New Roman Angsana New Browallia New Symbol Default Design Microsoft Equation 3.0 CS ChemDraw ... – PowerPoint PPT presentation
Number of Views:470
Avg rating:3.0/5.0
Title: Infrared Absorption Spectroscopy
1
Infrared Absorption Spectroscopy
2
IR Spectroscopy
- deal with the interaction of infrared radiation
with - matter
IR spectrum (T against Frequency)
- chemical nature and molecular structure of cpd
Applications
- organic materials
- polyatomic inorganic molecules
- organometallic compounds
3
IR region of the electromagnetic spectrum
- wavelength 770 nm to 1000 mm
- (wave number 12,900 to 10 cm-1)
IR region is often further subdivided into
three subregions
- Near-infrared region (nearest to the visible)
- Mid-infrared region
- Far-infrared region
4
Table Infrared Spectral Regions
Region
wavenumber Range, cm-1
Wavelength (l) Range, mm
Frequency (v) Range, Hz
Near
0.78 to 2.5
12800 to 4000
3.8x1014 to 1.2x1014
Middle
2.5 to 50
4000 to 200
1.2x1014 to 6.0x1012
Far
50 to 1000
200 to 10
6.0x1012 to 3.0x1011
Most used
2.5 to 15
4000 to 670
1.2x1014 to 2.0x1013
5
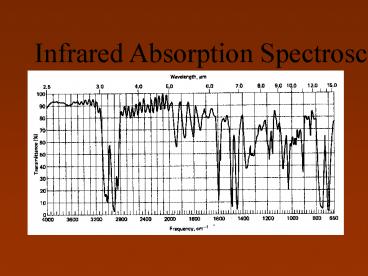
IR Spectrum
6
Mid-infrared region
1. Group-frequency region
- wavenumber 4000 to 1300 cm-1 (2.5 to 8 mm)
- functional group
2. Finger print region
- wavenumber 1300 to 650 cm-1
- ????????????????????????????????????
7
Infrared Spectrometry
- useful for quantitative analysis, although it is
- considerably more difficult to achieve accurate
and - precise results with IR spectrometry than with
- UV-visible methods
- Beers Law provides the basis of quantitative IR
- method as it does in UV-visible spectrophotometry
Electromagnetic radiation
UV-visible electronic
transition
infrared vibration,
rotation
8
Basis of Infrared Absorption
The IR spectrum can be obtained with gas-phase
or with condensed-phase molecules.
For gas-phase, molecules vibration-rotation
spectra are observed.
For condensed-phase, the rotaional structure is
lost.
Vibrational spectroscopy
9
Requirements for the absorption of IR radation
1. The natural frequency of vibration of the
molecules must equal the frequency of the
incident radiation
10
Types of Molecular Vibrations
IR Vibration of bonds
- Stretching
- Bending
Stretching vibration
?????????????????????????????????????????????? ???
????????????
- Symmetric stretching
- Asymmetric stretching
11
Methylene
Symmetric stretching
(2853 cm-1)
Asymmetric stretching
(2926 cm-1)
12
Bending vibration
????????????????????????????????
- Scissoring
- Rocking
- Wagging
- Twisting
13
In plane
Out of plane
Bending
14
Vibrational mode of methylene group
15
Number of Vibrational Modes
Nonlinear molecule
Fundamental vibrational modes 3N-6
Linear molecule
Fundamental vibrational modes 3N-5
16
Nonlinear molecule ?H2O
Vibrational modes 3(3) - 6 3
17
Linear molecule CO2
Vibrational modes 3N-5 3(3)-5 4
18
Molecular Vibration
A molecule is made up ofa number of atoms
joined by chemical bonds. Such atoms vibrate
about each other in the same way as weights held
together by springs
19
Hookes Law states that two masses joined by a
spring will vibrate such that
(1)
where the frequency (rad/sec), but
since
we have
(2)
20
where the frequency of vibration, k is
the force constant of the bond (N/cm), and
is the reduced mass, or
(3)
where M1 is the mass of one vibrating body, M2
the mass of the other. But is in cyles
per second (cps). During this time light travels
a distance measured in cm/sec (I.e., the speed
of light).
21
Therefore, if one divides by c, the result
is the number of cycle per cm. This is ,
the wavenumber of an absorption peak (cm-1) and
(4)
It can be deduced that
(5)
(6)
22
Example
Calculate the approximate wavenumber and
wavelength of the fundamental absorption peak
due to the stretching vibration of a carbonyl
group CO
The mass of the carbon atom in kg is given by
23
Similar, for oxygen
and the reduced mass m is given by
The force constant for the typical double bond is
about 1x103 N/cm. Substituting this value and m
into eq. (5) gives
24
The carbonyl stretching band is found
experimentally to be in the region of 1600 to
1800 cm-1 (6.3 to 5.6 mm)
25
Frequencies of various group vibrations in the
group frequency region and in fingerprint region
26
Instrumentation
Three distinct types of instruments employed for
IR absorption spectrometry
1. Dispersive instruments with a monochromator
are used in the mid-IR region for spectral
scanning and quantitative analysis
2. Fourier transform IR systems are widely
applied in the far-IR region and becoming quite
popular for mid-IR spectrometry
27
Instrumentation
3. Nondispersive instruments that use filters for
wavelength selection or an infrared-absorbing-gas
in the detection system are often used for gas
analysis at specific wavelength
28
Block diagram of IR spectrophotometer
readout
detector
source
sample
monochromator
Recorder XY plotter Printer
Grating Filter
Thermal D Thermocouple Thermopile Thermister Bolom
eter Pneumatic D Pyroelectric D
Nernst Glower Globar Incandescent wire source Hg
Arc
29
IR sources general
- an inert solid that is heated electrically to a
- temperature between 1500 and 2200 K
- (provide continuous radiant)
- the maximum radiant intensity at these
- temperatures occurs at between 5000 and 5900 cm-1
- (2 to 1.7 mm)
30
IR sources
The Nernst Glower (Continuous source)
- useful and inexpensive source
- rare earth oxides formed into a cylinder having
a - diameter of 1 to 2 mm and a length of perhaps 20
mm - platinum leads are sealed to the end of the
cylinder - to permit passage of electricity temperatures
between - 1200 and 2200 K result
- because of a negative temperature coefficient of
- resistance, it must be used with ballast resistor
in the - heating circuit to prevent burnout
31
IR sources
The Nernst Glower (Continuous source)
(cont.)
- it is rather fragile, and its lifetime depends
on the - operating temperature and the care taken in
handling it
32
IR sources
The Nernst Glower (Continuous source)
33
IR sources
The globar (continuous source)
- a silicon carbide rod, usually about 50 mm in
length - and 5 mm in diameter
- current through the globar causes the rod to
heat and - emit radiation at temperature exceeding 1000 oC
- the power consumption is normally higher than
that - of the Nernst Glower
- water cooling is needed to cool the metallic
electrodes - attached to the rod
- less convenient to use and more expensive
because - of the necessity for water cooling
34
IR sources
Incandescent wire source
- somewhat lower intensity but longer life than
- the Globar or Nernst glower
- a tightly wound spiral of nichrome wire heated
to - about 1100 K by an electrical current
- a rhodium-wire heater sealed in a ceramic
cylinder - has a similar properties as a source
35
IR sources
The Mercury arc
- for the far-infrared region of the spectrum (lgt
50 mm)
- provide sufficient energy for convenient
detection
- consist of a quartz-jacketed tube containing
mercury - vapour at a pressure greater than one atmosphere
- passage of electricity through the vapour forms
an - internal plasma source that provides continuous
- radiation in the far-infrared region
36
IR sources
The Mercury arc
37
IR sources
The Tungsten filament lamp
- the near-infrared region of
- 4000 to 12,800 cm-1
- (2.5 to 0.78 mm)
38
Infrared Detectors
General types of infrared detectors
1. Thermal Detectors
Dispersive spectrophotometer
2. Pyroelectric Detectors
3. Photoconducting Detectors
Fourier Transform multiplex instrument
39
Infrared Detectors
Thermal Detectors
- widely used in the IR region of the spectrum
- responses depends upon the heating
- effect of radiation
Problem
The problem of measuring infrared radiation
by thermal means is compounded by thermal noise
from surrounding
40
Infrared Detectors
Solution
Thermal detectors are usually encapsulated and
carefully shielded from thermal radiation
emitted by other nearby objects
41
Infrared Detectors
Thermal detectors Thermocouples
- a thermocouple is made by welding together at
- each end two wires made from different metals.
- If one welded joint (called the hot junction)
becomes - hotter than the other joint (the cold junction),
a small - electrical potential develops between the joints
42
Infrared Detectors
Thermal detectors Thermocouples
In IR spectroscopy, the cold junction is
carefully screened in a protective box and kept
at a constant temperature. The hot junction is
exposed to the IR radiation, which increases the
temperature of the junction. The potential
difference generated in the wires is a function
of the temperature difference between the
junctions and, therefore, of the intensity of IR
radiation falling on the hot junction.
43
Infrared Detectors
Thermal detectors Thermocouples
A well-designed thermocouple detector is
capable of responding to temperature difference
of 10-6 K. This figure corresponds to a
potential difference of about 6 to 8 mV/mW
To enhanced sensitivity, several
thermocouples may be connected in series to give
what a called a thermopile
44
Infrared Detectors
Thermal detectors Thermistor/Bolometer
A bolometer is a type of resistance
thermometer constructed of strips of metals such
as platinum or nickel, or from a mixture of
metal oxide the latter devices are sometimes
called thermistors. These materials exhibit a
relatively large change in resistance as a
function of Temperature.
The thermistor is normally placed in a bridge
circuit with a reference thermistor that is not
irradiated. The resistance can be measured by a
null-comparison method