Bell Ringer PowerPoint PPT Presentation
Title: Bell Ringer
1
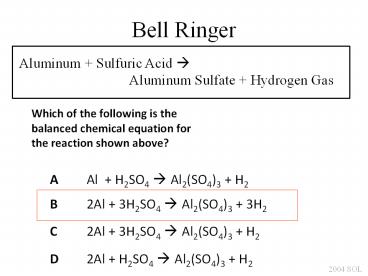
Bell Ringer
Aluminum Sulfuric Acid ?
Aluminum Sulfate
Hydrogen Gas
Which of the following is the balanced chemical
equation for the reaction shown above?
A Al H2SO4 ? Al2(SO4)3 H2
B 2Al 3H2SO4 ? Al2(SO4)3 3H2
C 2Al 3H2SO4 ? Al2(SO4)3 H2
D 2Al H2SO4 ? Al2(SO4)3 H2
2004 SOL
2
Chocolate Chip Cookies
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
3
Chocolate Chip Cookies
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
4
Chocolate Chip Cookies
- 2.25 flour
- 8 butter
- 0.5 shortening
- 0.75 sugar
- 0.75 brown sugar
- 1 salt
- 1 baking soda
- 1 vanilla
- 0.5 Egg Beaters
5
Chocolate Chip Cookies
- 2.25 cups
- 8 Tbsp
- 0.5 cups
- 0.75 cups
- 0.75 cups
- 1 tsp
- 1 tsp
- 1 tsp
- 0.5 cups
6
Chocolate Chip Cookies
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
7
Get on with it!
What does this have to do with CHEMISTRY?
8
2.25 cups flour 8 Tbsp butter 0.5 cups
shortening 0.75 cups sugar 0.75 cups brown
sugar 1 tsp salt 1 tsp baking soda 1 tsp
vanilla 0.5 cups Egg Beaters
unit
substance
coefficient
(a synthesis reaction)
9
Welcome to STOICHIOMETRY
- Ms. Besal
- 2/23/2006
10
What is Stoichiometry?
- The study of quantitative relationships within
chemical reactions - A balanced equation is the key to stoichiometry!
- Tools youll need for this chapter
- Writing proper formulas and balanced reactions
- Converting from mass to moles and vice versa
11
Lets Revisit the Cookies
For 1 batch
The Egg Beaters I have are close to expiring!
Id like to use the rest of them in this recipe.
I have 1.5 cups of Egg Beaters.
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
How many batches of cookies can I make with that
many Egg Beaters?
12
Lets Revisit the Cookies
For 1 batch
I have 1.5 cups of Egg Beaters.
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
How many batches of cookies can I make with that
many Egg Beaters?
1 batch cookies
1.5 cups E.B.
x
0.5 cups E.B.
3.0 batches of cookies
13
Lets Revisit the Cookies
For 1 batch
I have 1.5 cups of Egg Beaters.
- 2.25 cups flour
- 8 Tbsp butter
- 0.5 cups shortening
- 0.75 cups sugar
- 0.75 cups brown sugar
- 1 tsp salt
- 1 tsp baking soda
- 1 tsp vanilla
- 0.5 cups Egg Beaters
How much butter do I need to deplete (use up) the
Egg Beaters?
8 Tbsp butter
1.5 cups E.B.
x
0.5 cups E.B.
24 Tablespoons of butter
14
Back to Chemistry
- There are three types of stoichiometry problems
we will deal with today - Mole-Mole problems (1 conversion)
- Mass-Mole problems (2 conversions)
- Mass-Mass problems (3 conversions)
given
required
15
Baby Steps Mole-Mole Problems
- Step 1 Write a BALANCED EQUATION
- Step 2 Determine the mole ratio from the
coefficients in the equation. - Mole ratio moles of required substance
- moles of given substance
- Step 3 Set up the problem like a unit conversion
and solve!
16
Mole-Mole Problems
Example
2 H2O
2 H2 O2
How many moles of water can be formed from 0.5
mol H2?
2 mol H2O
0.5 mol H2
0.5 mol H2O
x
2 mol H2
17
Mole-Mole Practice
CuSO4
Al
Al2(SO4)3
Cu
Mole ratio
3 mol CuSO4
1. a.
0.5 mol Al
0.8 mol CuSO4
x
2 mol Al
1 mol Al2(SO4)3
b.
0.5 mol Al
0.3 mol Al2(SO4)3
x
2 mol Al
3 mol Cu
c.
0.5 mol Al
0.8 mol Cu
x
2 mol Al
18
Mole-Mole Practice
Ca
AlCl3
CaCl2
Al
3
2 mol AlCl3
2. a.
2.5 mol Ca
1.7 mol AlCl3
x
3 mol Ca
3 mol CaCl2
b.
2.5 mol Ca
2.5 mol CaCl2
x
3 mol Ca
2 mol Al
c.
2.5 mol Ca
1.7 mol Al
x
3 mol Ca
19
Mass-Mole Problems
- Step 1 Write a BALANCED EQUATION
- Step 2 Calculate the molar mass of your given
substance and convert from mass to moles - Step 3 Determine the mole ratio from the
coefficients in the equation - Step 4 Set up the conversion and solve!
20
Mass-Mole Problems
Example
2 H2O
2 H2 O2
How many moles of water can be formed from 48.0 g
O2?
2 mol H2O
1 mol O2
48.0 g O2
3.00 mol H2O
x
x
1 mol O2
32.00 g O2
21
Mass-Mole Practice
CuSO4
Al
Al2(SO4)3
Cu
Mole ratio
1 mol Al
3 mol CuSO4
1. a.
13.5 g Al
0.751 mol CuSO4
x
x
26.98 g Al
2 mol Al
1 mol Al2(SO4)3
1 mol Al
b.
13.5 g Al
x
0.250 mol Al2(SO4)3
x
2 mol Al
26.98 g Al
3 mol Cu
1 mol Al
c.
13.5 g Al
x
0.751 mol Cu
x
2 mol Al
26.98 g Al
22
Mass-Mole Practice
Ca
AlCl3
CaCl2
Al
3
2 mol AlCl3
1 mol Ca
2. a.
5.7 g Ca
x
0.095 mol AlCl3
x
3 mol Ca
40.08 g Ca
3 mol CaCl2
1 mol Ca
b.
5.7 g Ca
x
0.14 mol CaCl2
x
3 mol Ca
40.08 g Ca
2 mol Al
1 mol Ca
c.
5.7 g Ca
x
0.095 mol Al
x
3 mol Ca
40.08 g Ca
23
Mass-Mass Problems
Example
2 H2O
2 H2 O2
How many grams of water can be formed from 48.0 g
O2?
2 mol H2O
1 mol O2
18.02 g H2O
48.0 g O2
54.1 g H2O
x
x
x
1 mol O2
32.00 g O2
1 mol H2O
24
Mass-Mass Practice
CuSO4
Al
Al2(SO4)3
Cu
Mole ratio
1 mol Al
3 mol CuSO4
159.61 g CuSO4
1. a.
8.5 g Al
x
x
x
26.98 g Al
2 mol Al
1 mol CuSO4
75 g CuSO4
1 mol Al2(SO4)3
1 mol Al
342.14 g Al2(SO4)3
b.
8.5 g Al
x
x
x
2 mol Al
26.98 g Al
1 mol Al2(SO4)3
54 g Al2(SO4)3
25
Mass-Mass Practice
3 mol Cu
1 mol Al
63.55 g Cu
c.
8.5 g Al
x
x
x
2 mol Al
26.98 g Al
1 mol Cu
30. g Cu
26
Mass-Mass Practice
Ca
AlCl3
CaCl2
Al
3
2 mol AlCl3
1 mol Ca
133.33 g AlCl3
2. a.
1.9 g Ca
x
x
x
3 mol Ca
40.08 g Ca
1 mol AlCl3
4.2 g AlCl3
3 mol CaCl2
1 mol Ca
110.98 g CaCl2
b.
1.9 g Ca
x
x
x
3 mol Ca
40.08 g Ca
1 mol CaCl2
5.3 g CaCl2
27
Mass-Mass Practice
Ca
AlCl3
CaCl2
Al
3
2 mol Al
1 mol Ca
26.98 g Al
c.
1.9 g Ca
x
x
x
3 mol Ca
40.08 g Ca
1 mol Al
0.85 g Al
- For Mondays Quiz
- 2 Mass-Mass problems, 10 points each
- Watch for Significant Figures!
- Label EVERYTHING!